Glucagon superfamily peptides exhibiting g protein-coupled receptor activity
A technology for glucagon and coupled receptors, applied in the field of GPCR ligands
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
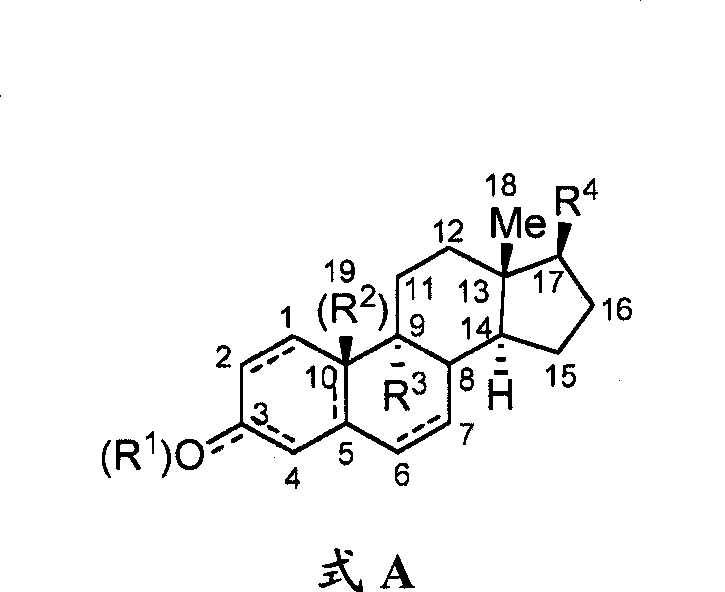
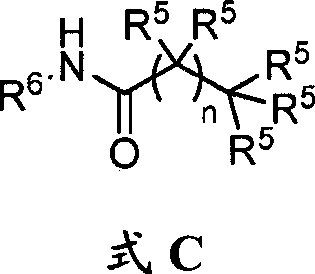
[0110] The present disclosure provides glucagon superfamily peptides conjugated to GPCR ligands. In some aspects, the GPCR ligand is capable of acting on a G protein-coupled receptor involved in metabolism or glucose homeostasis, and the conjugate provides a more favorable effect on metabolism or glucose homeostasis than either the peptide alone or the GPCR ligand alone. Excellent biological effect. Without being bound by the theory of the invention, GPCR ligands can be used to target glucagon superfamily peptides to specific types of cells or tissues; alternatively, glucagon superfamily peptides can be used to target GPCR ligands to cells Or the transport of the conjugate into the cell is facilitated, eg, by binding the peptide to a receptor that internalizes the conjugate.
[0111] The glucagon superfamily peptide conjugates of the present invention can be represented by the following formula:
[0112] Q-L-Y
[0113] wherein Q is a glucagon superfamily peptide, Y is a GPC...
Embodiment 1
[1445] Example 1: Synthesis of Peptide Fragments of Glucagon Superfamily Peptides
[1446] Material
[1447] Peptides are synthesized by any method known in the art. The following is an exemplary protocol. Peptides can be amidated at the C-terminus. MBHA (4-methylbenzhydrylamine polystyrene) resin can be used during peptide synthesis. MBHA resin, 100-180 mesh, 1% divinylbenzene (DVB) crosslinked polystyrene; loading 0.7-1.0mmol / g), Boc protected (tert-butyl carbamate) and Fmoc protected (9- Fluorenylmethylcarbamate) amino acids are commercially available from Midwest Biotech. Solid-phase peptide synthesis using Boc-protected amino acids can be performed in an Applied Biosystem 430A peptide synthesizer. Fmoc-protected amino acid synthesis can be performed using an Applied Biosystems Model 433 peptide synthesizer. In a particular embodiment, the following general peptide synthesis scheme using the Boc-chemistry strategy can be used
[1448] Peptide synthesis using Boc che...
Embodiment 2
[1467] The ability of each peptide to induce cAMP was measured in a firefly luciferase-based reporter assay. The induced cAMP production is directly proportional to the fragment of glucagon bound to the glucagon receptor or GIP receptor or GLP-1 receptor. HEK293 cells co-transfected with the receptor and luciferase genes linked to the cAMP response element were used in the bioassay.
[1468] Serum-deprived cells were cultured in Dulbecco's Modified Minimal Essential Medium (Invitrogen, Carlsbad, CA) supplemented with 0.25% bovine growth serum (HyClone, Logan, UT) for 16 hours, then incubated at 37°C, 5% CO 2Next, serial dilutions of glucagon fragments were incubated for 5 hours in 96-well poly-D-lysine-coated "Biocoat" plates (BD Biosciences, San Jose, CA). At the end of the incubation, 100 μL of LucLite Luminescent Substrate Reagent (PerkinElmer, Wellesley, MA) was added to each well. The plates were shaken briefly, incubated in the dark for 10 min, and the light output was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bmi | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com