Perovskite/metal composite oxide catalyst and preparation method thereof
A metal composite and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as insufficient performance and catalytic performance changes, and achieve improved catalytic performance The effect of responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method of catalyst provided by the invention comprises the steps:
[0013] (1) According to the molar ratio of metal elements in the general formula expression, the soluble salts of elements A and B are made into a solution with a total metal ion concentration of 0.1 to 1.0 mol / L, and the total molar number of A and B metals is 0.5 to 0.7 times Add an organic complexing agent to the solution, mix well, and heat to 50-80°C to form a wet gel.
[0014] (2) Raise the temperature of the wet gel to 100-150°C, heat the wet gel to gradually dry until it spontaneously ignites, grind the obtained ashes into powder, perform the first roasting at 300-500°C, and then bake at 700-900°C second roasting,
[0015] The step (1) of the above method is to prepare a wet gel. First, the soluble salts of elements A and B are formulated into a solution. The total metal ion concentration in the solution is preferably 0.1-0.8mol / L, and then an organic complexing agent is added t...
example 1
[0021] The composite oxides described in the present invention are prepared.
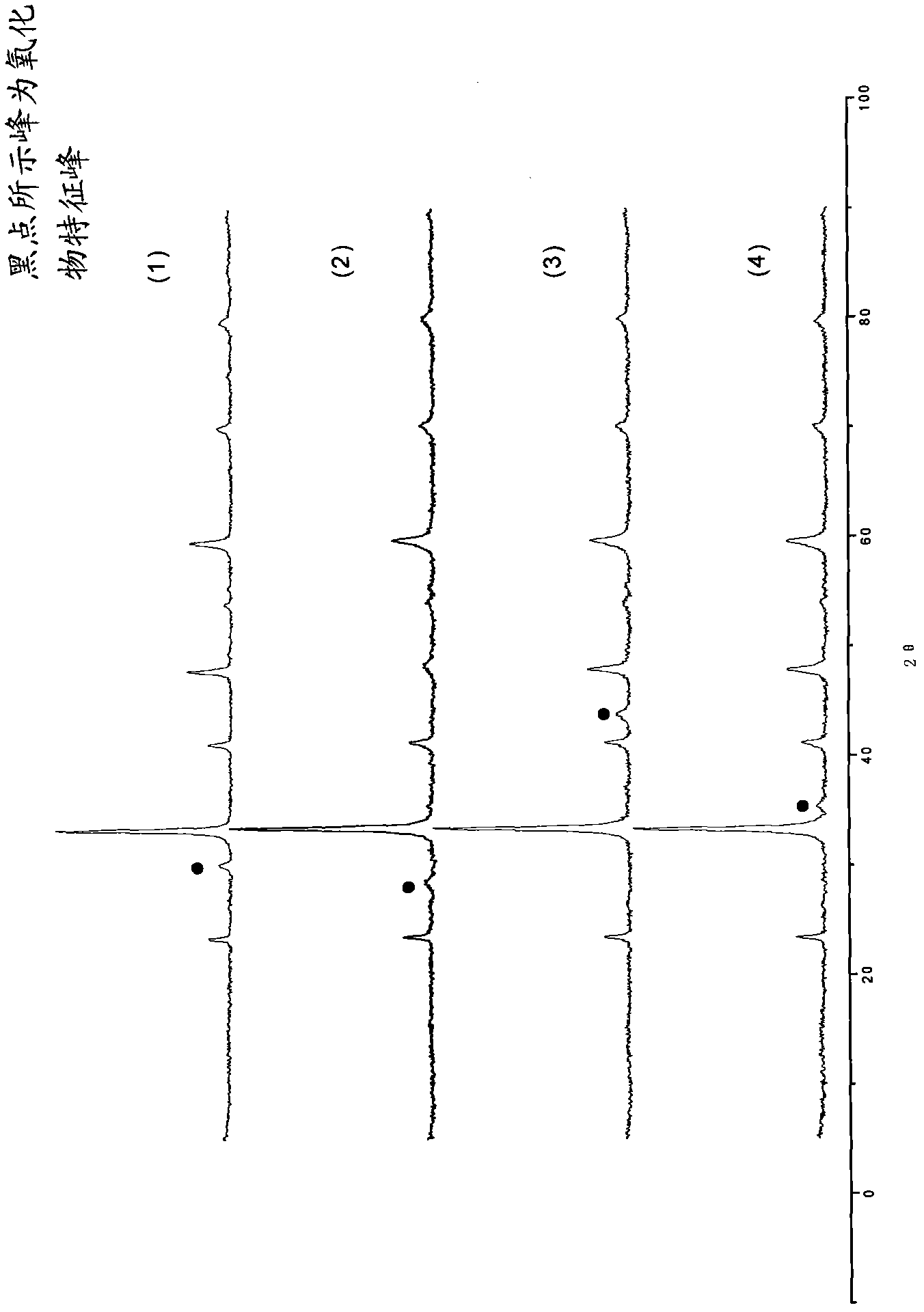
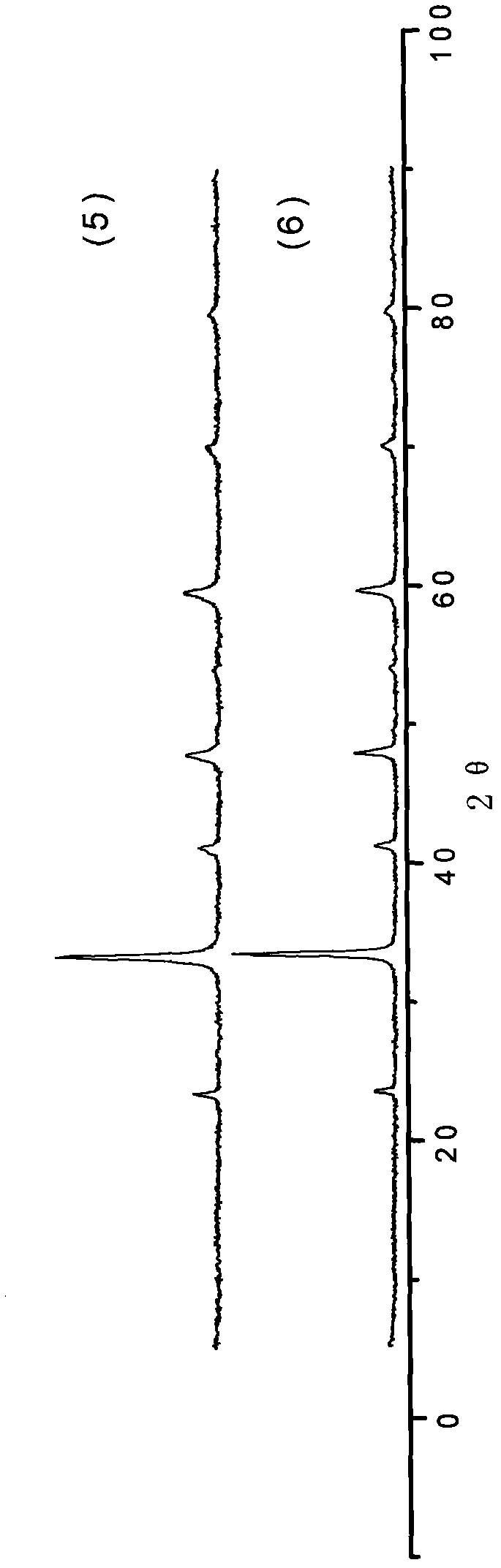
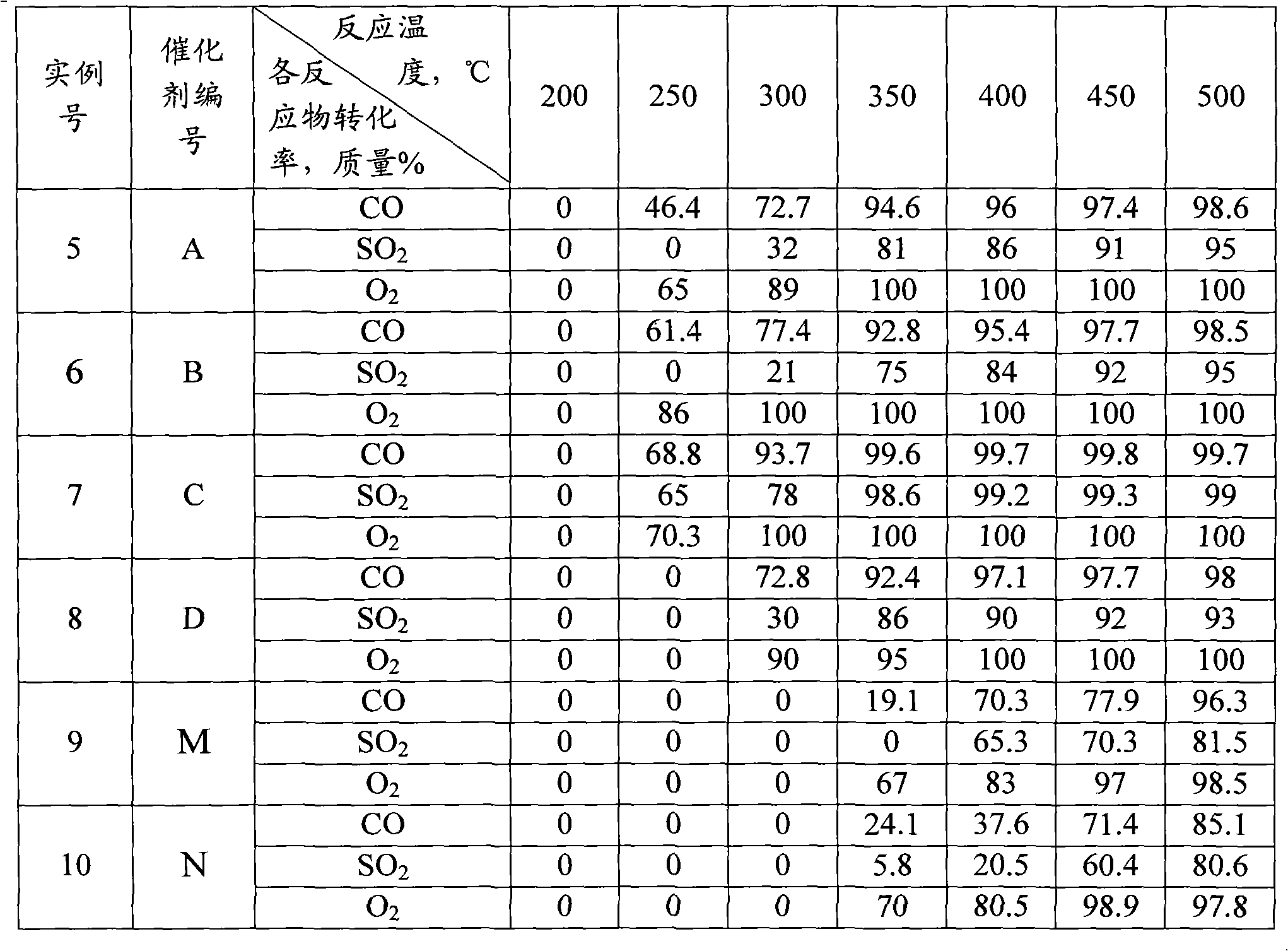
[0022] La(NO 3 ) 3 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O was dissolved in water at a molar ratio of 1.15:1 to form a mixed solution with a total metal ion concentration of 0.5 mol / L, and citric acid was added in an amount 0.6 times the total number of moles of metal ions, and stirred for 4 hours to dissolve. Heat the mixed solution to 70°C and stir until it becomes a wet gel, raise the temperature to 110°C, heat to make the gel gradually dry until it spontaneously ignites, grind the obtained ash into powder, bake at 400°C for 2 hours, press into tablets, and then heat at 800°C Roasting for 4 hours, the general formula is LaNiO 3 0.075La 2 o 3 The composite oxide of is catalyst A, and its XRD spectrum is shown in figure 1 curve (1). It can be seen from curve (1) that catalyst A mainly has LaNiO 3 Characteristic peaks of perovskite crystals with a small amount of La 2 o 3 The characteristic peak (...
example 2
[0024] Set Y(NO 3 ) 3 ·6H 2 O, Co(NO 3 ) 2 ·6H 2 O was dissolved in water at a molar ratio of 1.2:1 to form a mixed solution with a total metal ion concentration of 0.5 mol / L, and citric acid was added in an amount 0.6 times the total number of moles of metal ions, and stirred for 4 hours to dissolve. Heat the mixed solution to 70°C and stir until it becomes a wet gel, raise the temperature to 110°C, heat to make the gel gradually dry until spontaneous combustion, grind the obtained ash into powder, bake at 400°C for 2 hours, press into tablets, and then bake at 800°C 4 hours, the general formula is YCoO 3 0.1Y 2 o 3 The compound oxide of is catalyst B, and its XRD spectrum is shown in figure 1 Curve (2). It can be seen from curve (2) that catalyst B mainly has YCoO 3 Characteristic peaks of perovskite crystals with a small amount of Y 2 o 3 The characteristic peaks indicate the formation of composites of perovskite and metal oxides.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com