Method for synthesis of acetal/ketal in presence of acidic ionic liquid as catalyst?
An acidic ionic liquid, acetal technology, applied in the formation/introduction of ether group/acetal group/ketal group, chemical instruments and methods, preparation of organic compounds, etc. High requirements, complex preparation process and other problems, to achieve the effect of simple separation of catalyst and product, high yield and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
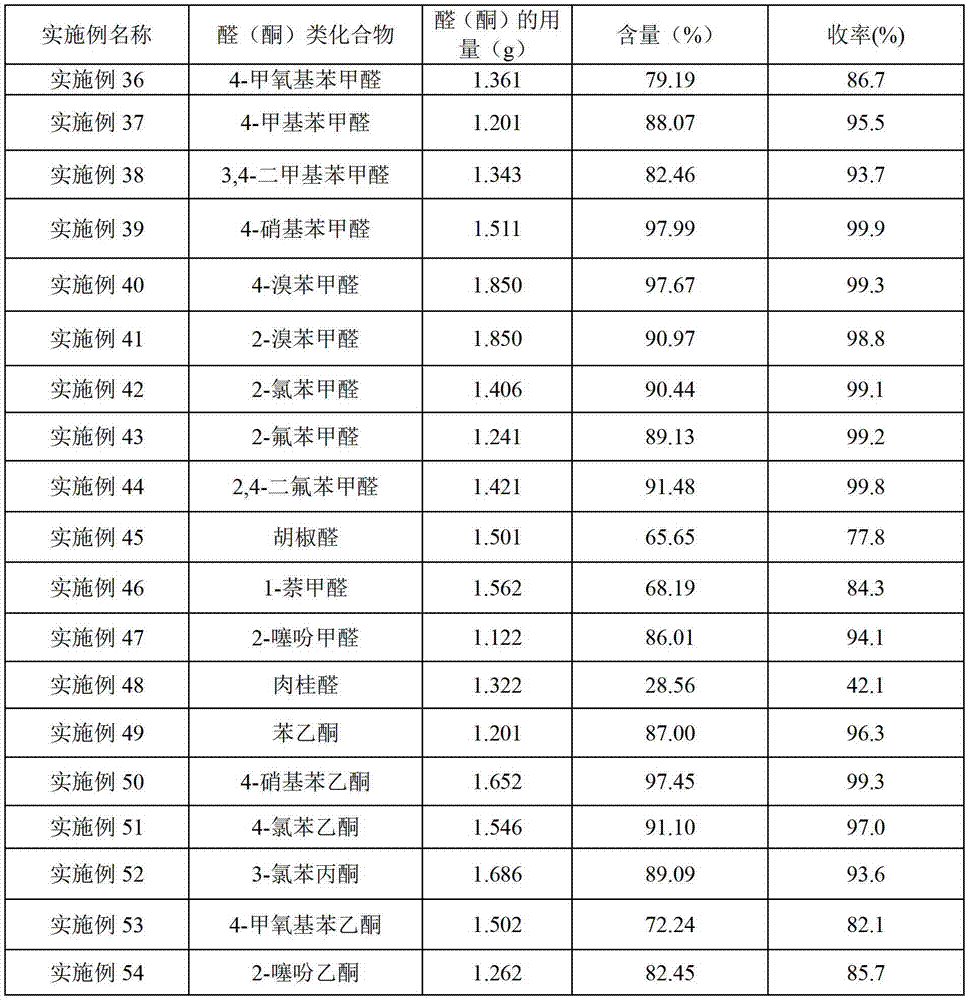
Examples
Embodiment 1
[0022] Example 1: Preparation of 3-(3-sulfonic acid) propylbenzothiazole bisulfate ionic liquid
[0023](1) Add benzothiazole (1.35g, 0.01mol) into a 25ml round-bottomed flask, and slowly add 1,3-propane sultone (1.464g, 0.012mol) dropwise under stirring conditions. After adding the raw materials, It was heated to 90°C for 4 hours to generate a large amount of white solid, which was washed three times with acetone and ether, filtered, and vacuum-dried at 80°C for 12 hours to obtain 2.47 g of white solid with a yield of 96.0%.
[0024] (2) Add 2.47g of the white solid obtained in step (1) into a 25ml round-bottomed flask, slowly add 0.94g (0.0096mol) of concentrated sulfuric acid (98wt%) under stirring in an ice bath, and add 2mL after the addition is complete distilled water at 80°C for 12 hours, the product was decompressed to remove water, washed three times with ethyl acetate, and dried in vacuum at 80°C for 12 hours to obtain 3.08 g of tan solid, namely 3-(3-sulfonic acid)...
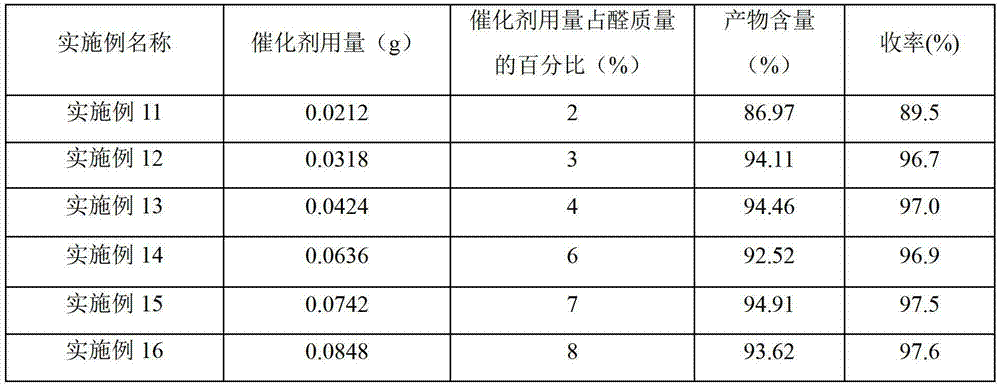
Embodiment 2~43-
[0025] Example 2-4 Preparation of 3-(3-sulfonic acid) propylbenzothiazole hydrochloride acidic ionic liquid
[0026] Different acids (shown in Table 1) were used to replace the concentrated sulfuric acid described in Example 1, and other conditions and operations were the same as in Example 1. The test result of embodiment 2~4 is listed in table 1.
[0027] Table 1 3-(3-sulfonic acid) propylbenzothiazole hydrochloride acidic ionic liquid with different anions
[0028]
Embodiment 5
[0029] Example 5: Preparation of immobilized 3-(3-sulfonic acid) propylbenzothiazole bisulfate ionic liquid
[0030] Mix 10mL of tetraethyl orthosilicate and 7mL of absolute ethanol into a 50mL round bottom flask, stir evenly at room temperature, heat to 60°C, add 2.00g of 3-(3-sulfonic acid prepared in Example 1 Acid) Propylbenzothiazole bisulfate ionic liquid ([C 3 SO 3 HBth] HSO 4 ), 2mL of concentrated hydrochloric acid (36%wt%), 5mL of distilled water, stirred for 10min, aged at 60°C for 24h to form a gel, and then vacuum-dried at 100°C for 12h to obtain 5.82g of 3-(3- Sulfonic acid) Propylbenzothiazole bisulfate acidic ionic liquid [(CH 2 ) 3 SO 3 HBth] HSO 4 / silica gel catalyst, the loading of its ionic liquid is 34%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com