Fused type prokaryotic expression vector and construction method and application thereof
A prokaryotic expression and fusion technology, applied in the field of fusion prokaryotic expression vectors and expression vectors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
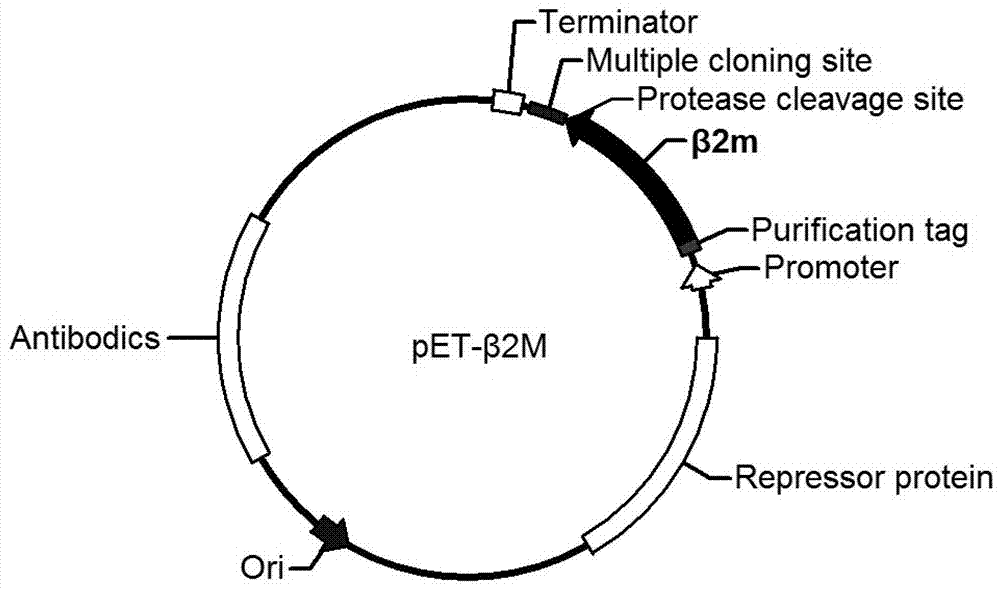
[0024] Embodiment 1 Contains the construction of the expression vector pET-β2M of β2M gene
[0025] 1) Synthesize the following nucleotide sequences with His purification tag, β2 microglobulin, enterokinase cleavage site, and polyclonal cleavage site:
[0026] CATCATCATCATCATCAT ATCCAGCGTACTCCAAAGATTCAGGTTTACTCACGTCATCCAGCAGAGAATGGAAAGTCAAATTTCCTGAATTGCTATGTGTCTGGGTTTCATCCATCCGACATTGAAGTTGACTTACTGAAGAATGGAGAGAGAATTGAAAAAGTGGAGCATTCAGACTTGTCTTTCAGCAAGGACTGGTCTTTCTATCTCTTGTACTACACTGAATTCACCCCCACTGAAAAAGATGAGTATGCCTGCCGTGTGAACCATGTGACTTTGTCACAGCCCAAGATAGTTAAGTGGGATCGAGACATG GACGACGACGAC AAG GGATCCGAGCTCCGTCGACAAGCTTGCGGCCGC (SEQ ID No. 2)
[0027] 2) In primer 1: 5'GTAC CCATGG GCCATCATCATCATCATCATAT3' (the underlined part is the Nco I recognition site)
[0028] Primer 2: 5'GTAC GCGGCCGC Under the guidance of AAGCTTGTCGACGGAGCTCG3' (the underlined part is the Not I recognition site), PCR was performed using the sequence in step 1) as a template, and the following nucleot...
Embodiment 2
[0031] Example 2 Construction of the expression vector pET-β2M-Vpr containing the Vpr protein gene of HIV and the β2M gene
[0032] 1) Synthesize the following Vpr gene sequence
[0033]ATGGAACAAGCCCCAGAAGACCAAGGGCCACAGAGGGAGCCACACAATGAATGGACACTAGAGCTTTTAGAGGAGCTTAAGAATGAAGCTGTTAGACATTTTCCTAGGATTTGGCTCCATGGCTTAGGGCAACATATCTATGAAACTTATGGGGATACTTGGGCAGGAGTGGAAGCCATAATAAGAATTCTGCAACAACTGCTGTTTATCCATTTCAGAATTGGGTGTCGACATAGCAGAATAGGCGTTACTCAACAGAGGAGAGCAAGAAATGGAGCCAGTAGATCC(SEQ ID No.3)
[0034] 2) Primer 1: 5'GTAC GGATCC ATGGAACAAGCCCCAGAAGA3' (the underlined part is BamH I recognition site)
[0035] Primer 2: 5'GTAC GCGGCCGC TTAGGATCTACTGGCTCCATTTC (the underlined part is Not I recognition site 1) was used as a guide, and the gene in step 1) was used as a template to perform PCR to obtain the following Vpr gene with Bam H I and Not I recognition sites:
[0036] GTAC GGATCC ATGGAACAAGCCCCAGAAGACCAAGGGCCACAGAGGGAGCCACACAATGAATGGACACTAGAGCTTTTAGAGGAGCTTAAGAATGAAGCTGTTAGACATT...
Embodiment 3
[0038] Expression and separation and purification of the Vpr protein of embodiment 3HIV
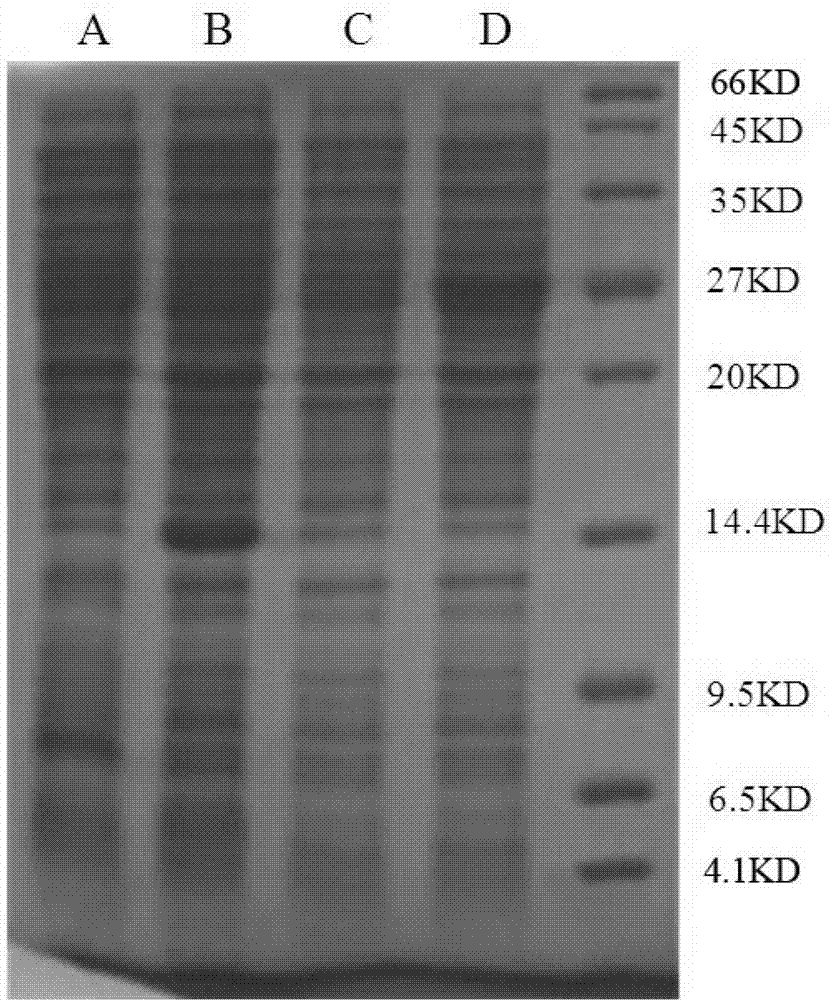
[0039] 1) The expression plasmid pET-β2M-Vpr obtained in Example 2 was treated with CaCl 2 Escherichia coli BL21 was transformed by method, and LB solid medium containing 100ug / ml kanamycin was used for selection. The Vpr expression strain was obtained and named pET-β2M-Vpr / BL21.
[0040] 2) Pick a single colony and culture it with LB liquid medium containing 100ug / ml kanamycin at 37°C and 220rpm for about 16 hours, then transfer 1% to fresh LB containing 100ug / ml kanamycin The liquid medium was shaken at 37°C and 220rpm for about 3 hours until OD600≈0.6, and IPTG was added to a final concentration of 1mM, and the shaking was continued for 3 hours.
[0041] 3) Centrifuge at 10,000 rpm at 4°C for 10 minutes to collect bacteria. Resuspend the bacteria in PBS, collect the bacteria by centrifugation for 10 minutes, resuspend the bacteria with 20mM pH8.0 Tris-HCl, 5mM EDTA, and sonicate the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com