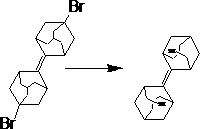
Tetracyclic decene dimer synthesis method
A tetracyclodedecane dimer and synthesis method technology, applied in the direction of chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problem of serious injury to operators, high toxicity of chemical reagents, tetracyclodedecane The problem of high cost of dimer synthesis achieves the effect of increasing yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
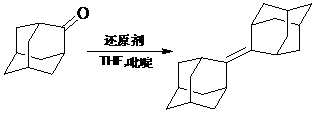
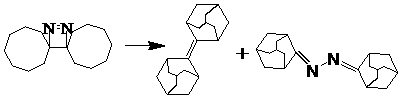
[0027] Add 100 ml of anhydrous tetrahydrofuran into a 500 ml three-necked flask, protect it with argon, and keep the temperature at 0°C. Add 11.38 g (0.060 mol) of titanium tetrachloride dropwise within 30 minutes with a horizontal pressure funnel, and stir rapidly; then batch A total of LiAlH was added 4 5.31 g (0.140 mol) g, after the dropwise addition, remove the cooling device, stir at room temperature for 30 minutes, then heat to 60-70°C and reflux for 30 minutes. After reflux, cool down to 0°C again, add 4.80 g (0.0607 mol) of pyridine dropwise; then add 120 ml of tetrahydrofuran solution of 10.00 g (0.0667 mol) tetracyclodecanone dropwise, remove the cooling device, and heat to 60-70 ℃, reflux reaction for 24 hours (argon protection). After the reaction, cool to room temperature, add 300 ml of newly prepared 10% potassium carbonate solution dropwise, filter, wash the filter cake twice with anhydrous ether, then combine the filtrates, wash with distilled water (3×50 ml...
Embodiment 2
[0029] Add 100 ml of anhydrous tetrahydrofuran to a 500 ml three-neck flask, protect it with argon, and keep the temperature at 0°C. Add 13.77 g (0.0726 mol) of titanium tetrachloride dropwise within 30 minutes with a horizontal pressure funnel, and stir rapidly; then batch A total of LiAlH was added 4 5.81 g (0.153 mol), after the dropwise addition, remove the cooling device, stir at room temperature for 30 minutes, then heat to 60-70°C and reflux for 30 minutes. After reflux, cool down to 0°C again, add 4.89 g (0.0618 mol) of pyridine dropwise; then add 120 ml of tetrahydrofuran solution of 10.00 g (0.0667 mol) tetracyclodecanone dropwise, remove the cooling device, and heat to 60-70 °C, reflux for 24 hours (argon protection). After the reaction, cool to room temperature, add 300 ml of newly prepared 10% potassium carbonate solution dropwise, filter, wash the filter cake twice with anhydrous ether, then combine the filtrates, wash with distilled water (3×50 ml), and use th...
Embodiment 3
[0031]Add 100 ml of anhydrous tetrahydrofuran into a 500 ml three-neck flask, protect it with argon, and keep the temperature at 0°C. Add 16.45 g (0.0867 mol) of titanium tetrachloride dropwise within 30 minutes with a horizontal pressure funnel, and stir rapidly; then batch A total of 6.34 g (0.167 mol) of LiAlH4 was added each time. After the dropwise addition, the cooling device was removed, stirred at room temperature for 30 minutes, and then heated to 60-70°C for 30 minutes under reflux. After reflux, cool down to 0°C again, add 5.01 g (0.0634 mol) of pyridine dropwise; then add 120 ml of tetrahydrofuran solution of 10.00 g (0.0667 mol) tetracyclodecanone dropwise, remove the cooling device, and heat to 60-70 °C, reflux for 24 hours (argon protection). After the reaction, cool to room temperature, add 300 ml of newly prepared 10% potassium carbonate solution dropwise, filter, wash the filter cake twice with anhydrous ether, then combine the filtrates, wash with distilled ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com