Nicotine compound containing double benzene and preparation method and use thereof
An aniline compound and compound technology, applied in the field of bisphenyl-containing nicotinic compounds and their preparation, can solve the problems of reduced pest killing rate and pest resistance, and achieve strong killing activity, high-efficiency killing, Broad-spectrum insecticidal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] A preparation method of a bisphenyl-containing nicotinic compound of the present invention comprises the following steps:
[0063] ① Add the aniline compound with the general structural formula A and the halide benzyl compound with the general structural formula B into the solvent in a molar ratio of 1-2:1, then add the acid-binding agent, and stir at 20-150°C React for 6-12h, filter to remove the solid, wash with hydrochloric acid to remove the remaining aniline compound A in the filtrate, and the remaining liquid is obtained by column chromatography to obtain intermediate II,
[0064] ;
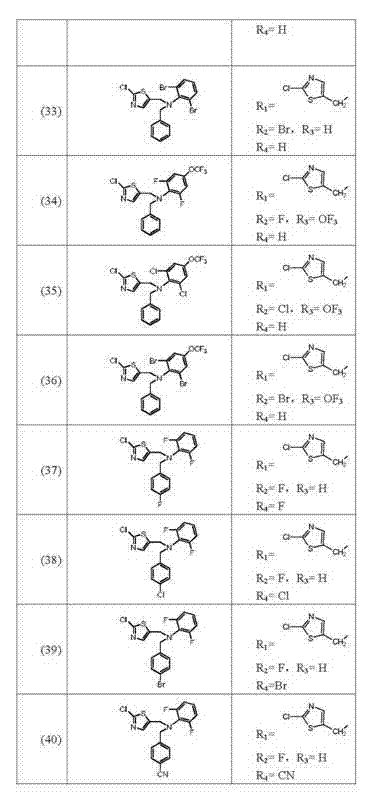
[0065]②The intermediate II obtained in step ① and 2-chloro-5-chloromethylpyridine or 2-chloro-5-chloromethylthiazole are sequentially added to the solvent in a molar ratio of 1:1, and the bound The acid agent is added to the reaction solution in batches within 5-8 hours. After the addition, the reaction solution is poured into water, and the solid is precipitated. The solid is obt...
Embodiment 1
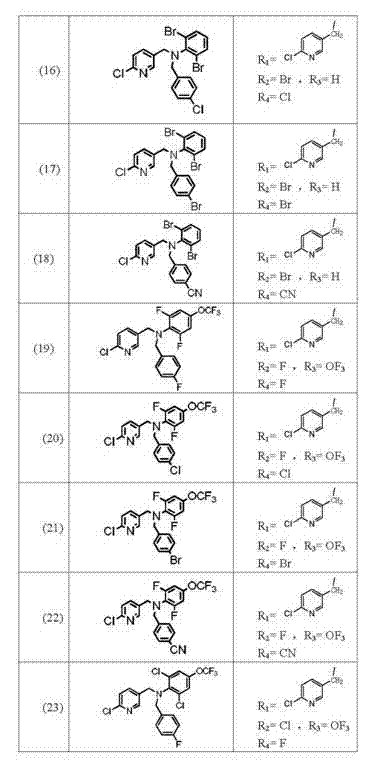
[0096] Example 1, the specific preparation steps of bisphenyl-containing nicotinic compound (1) are as follows:
[0097] ① Add 0.1mol 2,6-difluoroaniline and 0.1mol benzyl chloride to 300ml methanol in turn, add 40g (0.1mol) sodium hydroxide, stir and react at 20°C for 12h, filter to remove solids, and wash the filtrate with hydrochloric acid Wash to remove the remaining 2,6-difluoroaniline, and the remaining liquid is obtained by column chromatography of ethyl acetate:petroleum ether=6:4 to obtain intermediate II;
[0098] ② Add the intermediate II obtained in step ① and 0.1mol 2-chloro-5-chloromethylpyridine to 300ml methanol in sequence, and add 40g (0.1mol) sodium hydroxide to the reaction solution in 4 batches within 8 hours at 30°C After the addition, the reaction solution was poured into 1000ml of water, the solid was precipitated, and the solid was obtained by filtration, and 29.25g of white solid was obtained by column chromatography of ethyl acetate:petroleum ether=9...
Embodiment 2
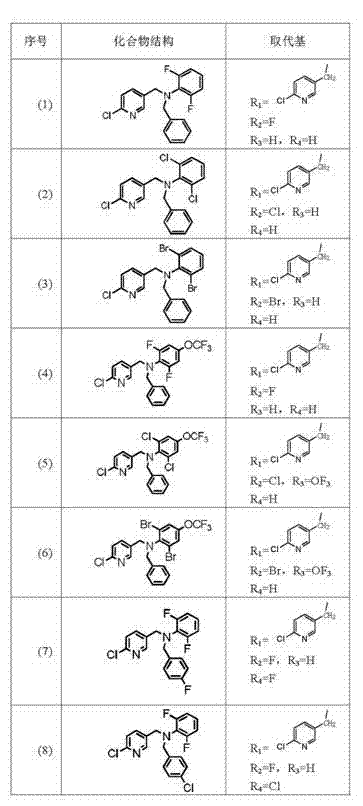
[0099] Example 2, the specific preparation steps of the bisphenyl-containing nicotinic compound (11) are as follows:
[0100] ① Add 0.2mol 2,6-dichloroaniline and 0.1mol p-fluorobenzyl bromide to 1500ml propanol in turn, add 138.19g (1mol) potassium carbonate, stir and react at 150°C for 6h, filter to remove the solid, and use the filtrate Wash with hydrochloric acid to remove the remaining 2,6-dichloroaniline, and the remaining solution is subjected to column chromatography of ethyl acetate:petroleum ether=7:3 to obtain intermediate II;
[0101] ② Add the intermediate II obtained in step ① and 0.1mol 2-chloro-5-chloromethylpyridine to 1500ml propanol in sequence, and add 138.19g (1mol) potassium carbonate to the reaction solution in 4 batches within 5h at 100°C After the addition, the reaction solution was poured into 1000ml of water, the solid was precipitated, and the solid was obtained by filtration, and 34.28g of white solid was obtained by column chromatography of ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com