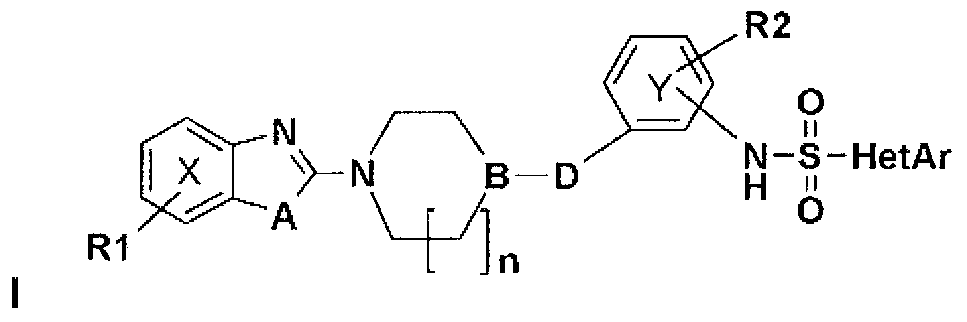
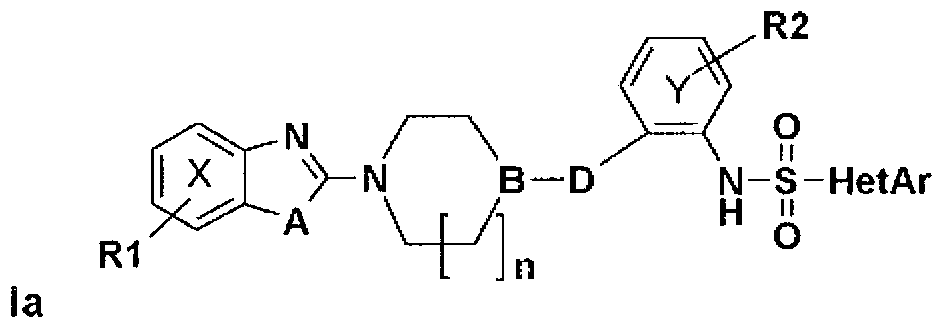
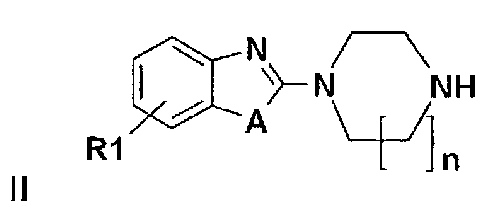
Derivatives of heteroarylsulfonamides, their preparation and their application in human therapy
A technology of sulfonamide and phenyl, applied in the field of heteroarylsulfonamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0271] Example 1 : N-(2-(4-(6-fluorobenzo[d]oxazol-2-yl)piperazine-1-carbonyl)phenyl)-5-(trifluoromethyl)pyridine-2-sulfonamide (1)
[0272]
[0273] Compound 1 was prepared using Synthetic Method 1: In the presence of 1H-benzo[d][1,3]oxazine-2,4-dione (1.83 g, 11.3 mmol) and DMAP (138 mg, 1.13 mmol), the Intermediate 3a (2.5 g, 11.30 mmol) was taken up in 25 mL THF and 5 mL DMF, and the mixture was stirred at ambient temperature overnight. After concentration to dryness, the residue was taken up in water and extracted with AcOEt. After drying the organic phase and concentrating to dryness, the residue obtained was purified by flash chromatography on silica gel (CH 2 Cl 2 -MeOH, gradient 100-0 to 95-5 over 40 minutes). 0.47 g of solid were obtained (yield: 32%). The obtained solid was then placed in 3 mL of dichloromethane in the presence of 0.119 g (0.48 mmol) of intermediate 4a and 39 μL of pyridine. The mixture was then stirred overnight at ambient temperature, t...
Embodiment 2
[0277] Example 2 : N-(2-(4-(6-fluorobenzo[d]thiazol-2-yl)piperazine-1-carbonyl)phenyl)-pyridine-2-sulfonamide (2)
[0278]
[0279] Compound 2 was prepared using Synthetic Method 1 using Intermediate 3e (yield: 87%) for the first step and Intermediate 4b (yield: 30%) for the second step.
[0280] TLC silica gel 60F254Merck, CH 2 Cl 2 -MeOH:95-5, Rf=0.58.
[0281] NMR 1 H(CDCl 3 ) ppm: 8.65(d,1H), 8.58(s,1H), 8.03(d,1H), 7.89(t,1H), 7.77(d,1H), 7.50(m,1H), 7.39(m,3H ),7.12(m,3H),3.62(m,8H).
[0282] MS(+ESI)m / z498(MH+)
Embodiment 3
[0283] Example 3 : N-(2-(4-(6-bromobenzo[d]thiazol-2-yl)piperazine-1-carbonyl)phenyl)-pyridine-2-sulfonamide (3)
[0284]
[0285] Compound 3 was prepared using Synthetic Method 1 using intermediate 3f for the first step (quantitative yield) and intermediate 4b for the second step (yield: 80%).
[0286] TLC silica gel 60F254Merck, CH 2 Cl 2 -MeOH:95-5, Rf=0.60.
[0287] mp=102℃
[0288] NMR 1 H(CDCl 3 )ppm:8.66(d,1H),8.56(s,1H),8.03(d,1H),7.88(t,1H),7.75(m,2H),7.41(m,4H),7.16(m,2H ),3.64(m,8H).
[0289] MS(+ESI)m / z558(MH+)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com