Method for detecting digestive tract pathogens
A pathogen and digestive tract technology, applied in the field of pathogenic microorganism molecular biology, can solve the problems of difficult clinical promotion, complicated operation, low degree of automation, etc., and achieve the effects of simple reagent consumables, stable reaction system, and less environmental pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] In the present embodiment, a method for detecting two kinds of pathogenic plasmids (Listeria monocytogenes, Enterovirus 71) is provided, comprising the steps of:
[0057] (1) Obtain the amplified product of the target sequence of the pathogen plasmid to be tested by PCR amplification. The amplification primer set used is shown in Table 1 (the corresponding primer combination in the reaction system 1 or 2 where the pathogen to be detected is located), wherein each primer needs to be prepared with a 10-base tag sequence (acgttggatg) at its 5' end ), the purpose is to increase the molecular weight of the primer sequence to exclude its influence in subsequent steps.
[0058] The reaction system used in PCR amplification is as follows, and all reagents are purchased from Sequenom Company:
[0059]
[0060]The PCR reaction conditions were: 94°C for 15 minutes; denaturation at 94°C for 20 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 1 minute, and a tota...
Embodiment 2
[0079] In this embodiment, a detection method for a clinically collected pathogen mixed infection sample is provided, including the following steps:
[0080] (1) Carry out preliminary treatment to the sample collected clinically, use the kit (QIAGEN company) to extract pathogen DNA and RNA, utilize the kit purchased from Takara company The RT reagent Kit reverse-transcribes the extracted RNA sample into cDNA.
[0081] Steps (2)-(6) are the same as steps (1)-(5) in Example 1, except that the template DNA used in the PCR amplification reaction system is the clinical sample DNA prepared in the above step (1) and cDNA, and the amplification primers and extension primers used are the amplification primers and extension primers corresponding to reaction system 2.
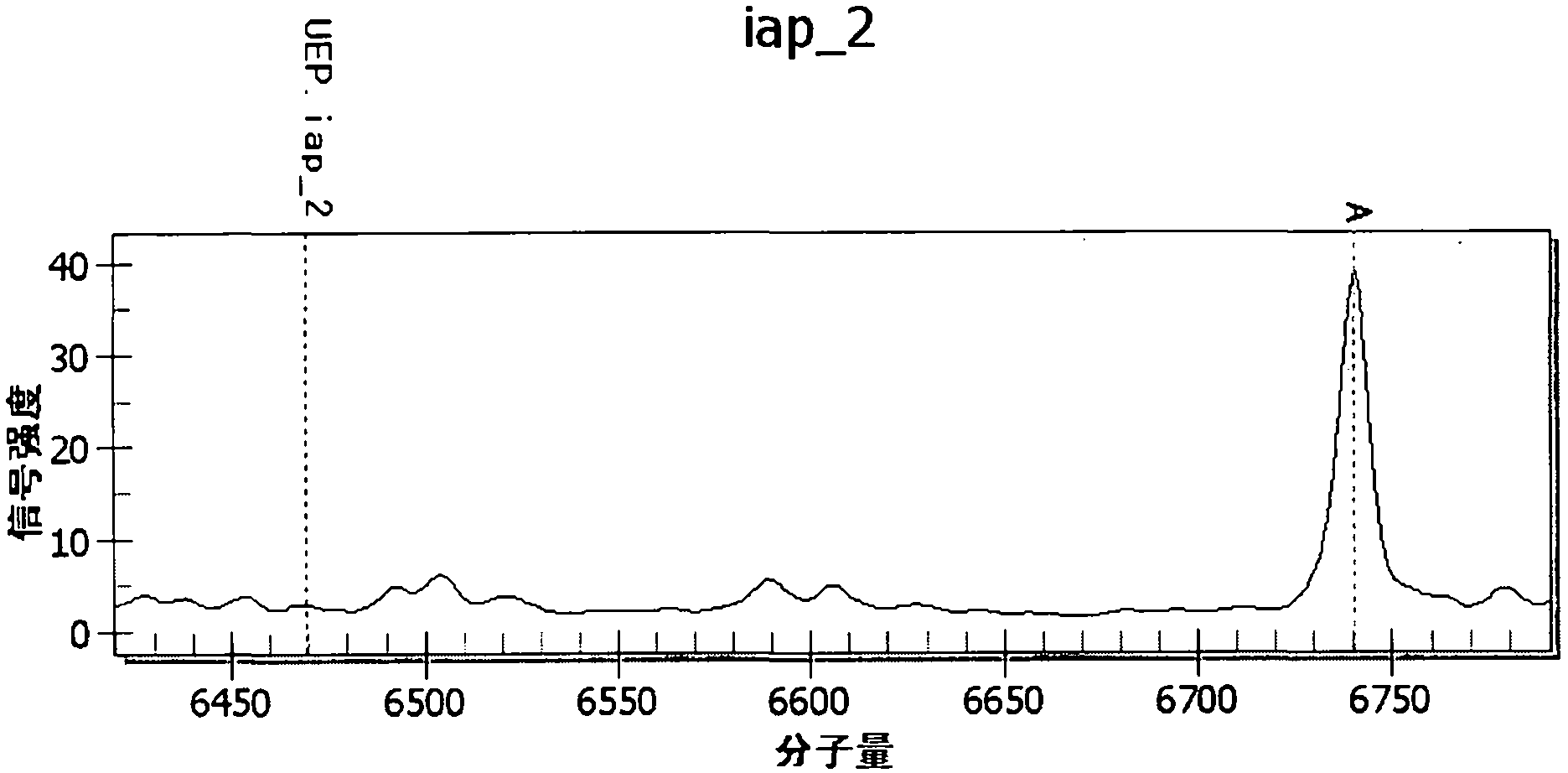
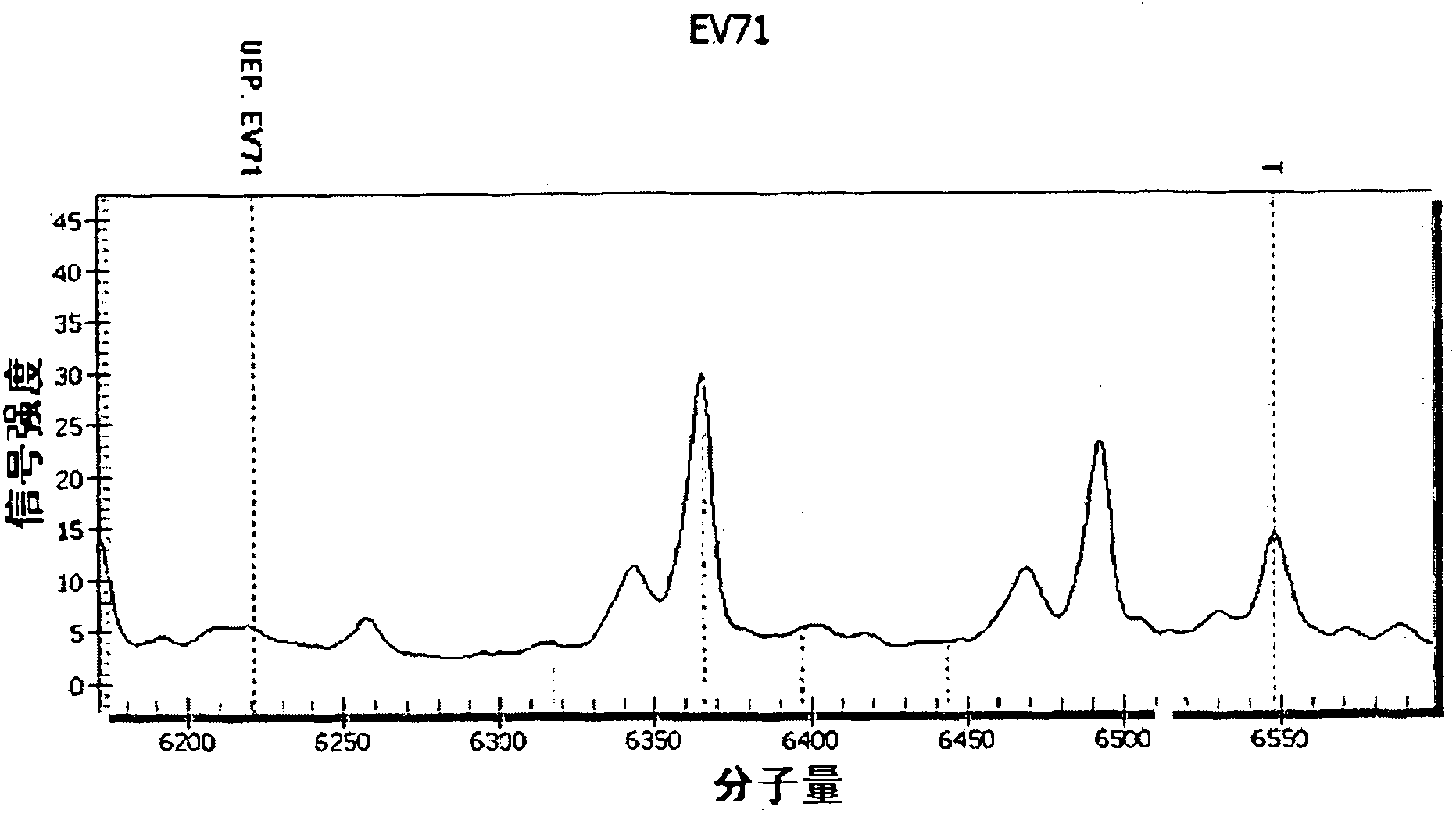
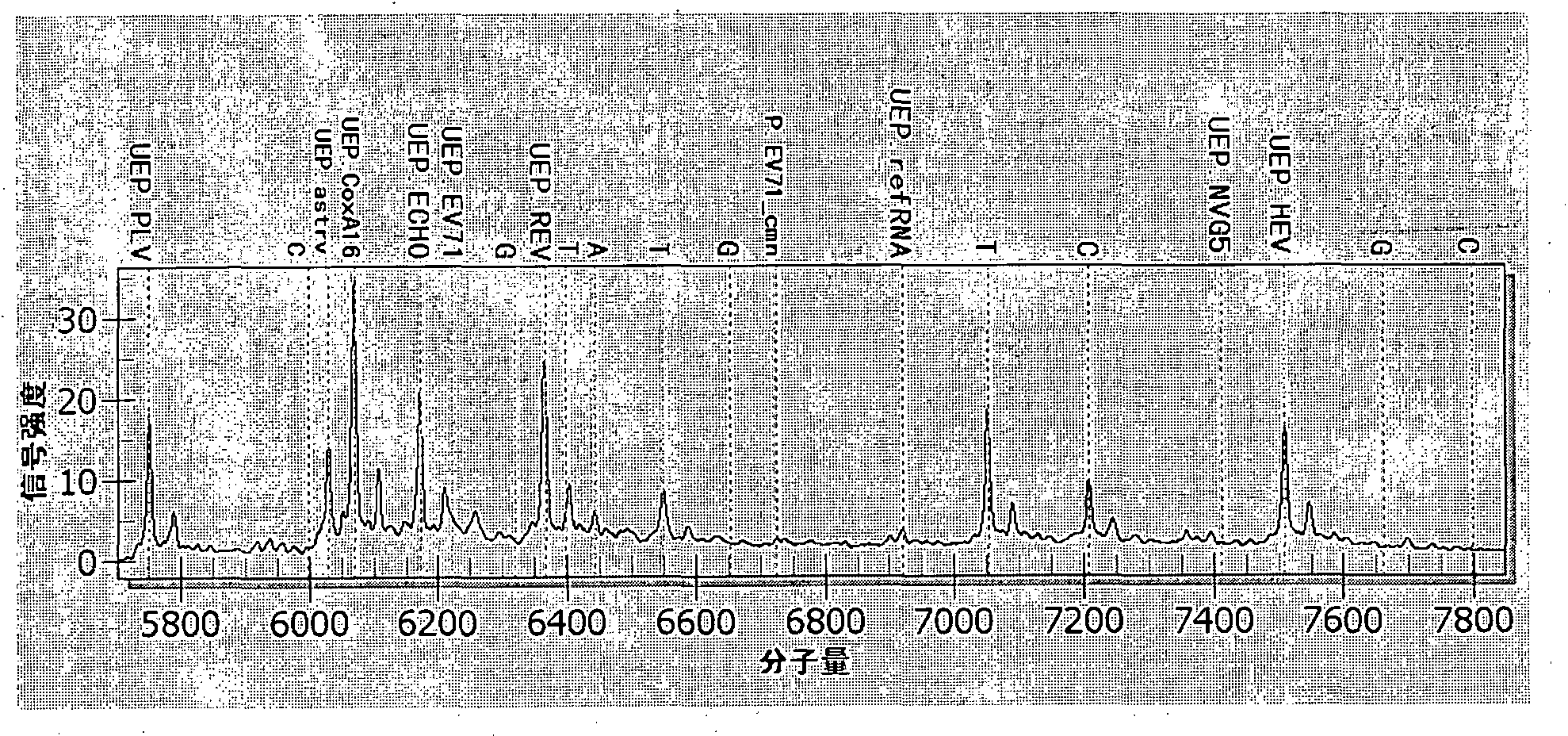
[0082] The following combination image 3 , to analyze and explain the test results of the above-mentioned pathogen mixed infection samples.
[0083] image 3 It is the mass spectrometry detection peak diagram when u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com