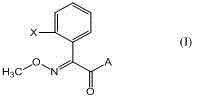
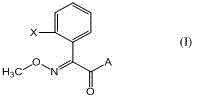
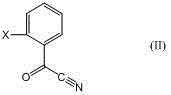
Method for preparing (E)-2-(2-substituted phenyl)-2-methoxyimino acetic acid derivative
A technology of methoxyiminoacetic acid and derivatives, applied in oxime preparation, organic chemistry and other directions, can solve the problems of harsh operating conditions, easy leakage of hydrogen chloride gas, and high corrosiveness of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1. Synthesis of (E)-methyl 2-(2-methylphenyl)-2-methoxyiminoacetate.
[0060] ⑴. Dissolve 146.6g (99%, 1.0mol) of o-toluoylnitrile in 200mL of hexane for later use. A 1000mL four-necked reaction flask is equipped with a mechanical stirrer, a reflux condenser, a thermometer and a dropping funnel, and then Put the above solution into the reaction bottle, start stirring, and use refrigerated brine to cool down, so that the temperature in the bottle drops below 20°C, and control it below 20°C, add methanol 150g (99%, 4.64mol) and sodium bromide 21g (99%, 0.2mol), after stirring for 30 minutes, control the temperature below 35°C and add dropwise an 85% sulfuric acid solution made of 400g (98%, 4mol) of sulfuric acid and 61mL of water, and add it in about 30 minutes, then maintain the temperature at 35°C React at ~40°C for 3 hours, track and analyze with gas chromatography, after the raw material benzoyl nitrile is less than 2%, heat it with an electric heating mantle...
Embodiment 2
[0065] Example 2, Synthesis of (E)-2-(2-chloromethylphenyl)-2-methoxyiminoacetic acid methyl ester
[0066] ⑴. Dissolve 181.5g (99%, 1.0mol) of o-chloromethylbenzoyl nitrile in 200mL cyclohexane for later use. A 1000mL four-necked reaction flask is equipped with mechanical stirring, reflux condenser, thermometer and dropwise Funnel, then add the above solution into the reaction bottle, start stirring, use refrigerated brine to cool, make the temperature in the bottle drop below 20°C, and control it below 20°C Add methanol 150g (99%, 4.64mol) and sodium chloride 23g (99%, 0.4mol), after stirring for 30 minutes, control the temperature below 35°C and add dropwise an 85% sulfuric acid solution made of 400g (98%, 4mol) of sulfuric acid and 61mL of water, and add it in about 30 minutes, then Keep the temperature between 35 and 40°C for 3 hours, and use GC to track and analyze. After the raw material benzoyl nitrile is less than 2%, heat it with an electric heating mantle, and slowl...
Embodiment 3
[0071] Example 3, Synthesis of (E)-2-[2-(2-methylphenoxymethyl)phenyl]-2-methoxyiminoacetic acid methyl ester (Kresstrobin)
[0072] ⑴. Dissolve 192.3g (98%, 0.75mol) of 2-(2-methylphenoxymethyl)benzoyl nitrile in 200mL dichloroethane for later use, and install mechanical stirring on a 1000mL four-necked reaction flask , a reflux condenser, a thermometer and a dropping funnel, then add the above solution into the reaction flask, start stirring, and use frozen brine to cool down the temperature in the bottle to below -5°C, and control it below -5°C by adding methanol 150g ( 99%, 4.64mol) and sodium bromide 39g (99%, 0.375mol), after stirring for 30 minutes, control the temperature below 15°C and dropwise add 85% of sulfuric acid 300g (98%, 3.0mol) and water 45mL Add the sulfuric acid solution in about 30 minutes, then keep the temperature between 25-30°C for 5 hours, follow up and analyze it with gas chromatography, after the raw material benzoyl nitrile is less than 2%, heat i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com