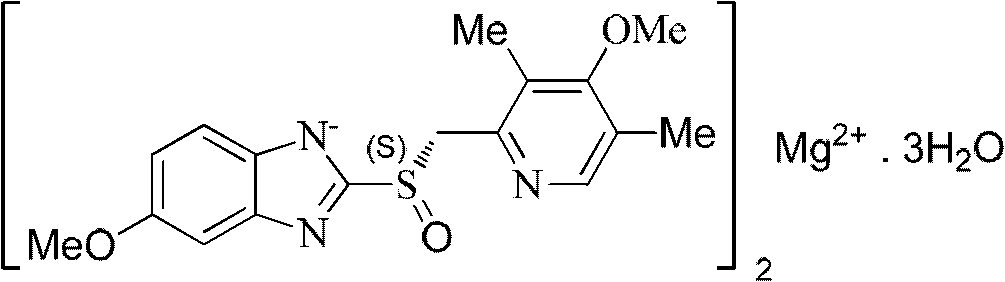
Esomeprazole magnesium dihydrate preparation method
A technology for esomeprazole magnesium dihydrate and esomeprazole magnesium trihydrate, applied in the field of chemical synthesis of pharmaceutical compositions, can solve the problems of toxic chlorinated solvents, low yield and the like, and achieve product crystal form Single, reproducible, easy-to-use results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1 Preparation of esomeprazole magnesium trihydrate
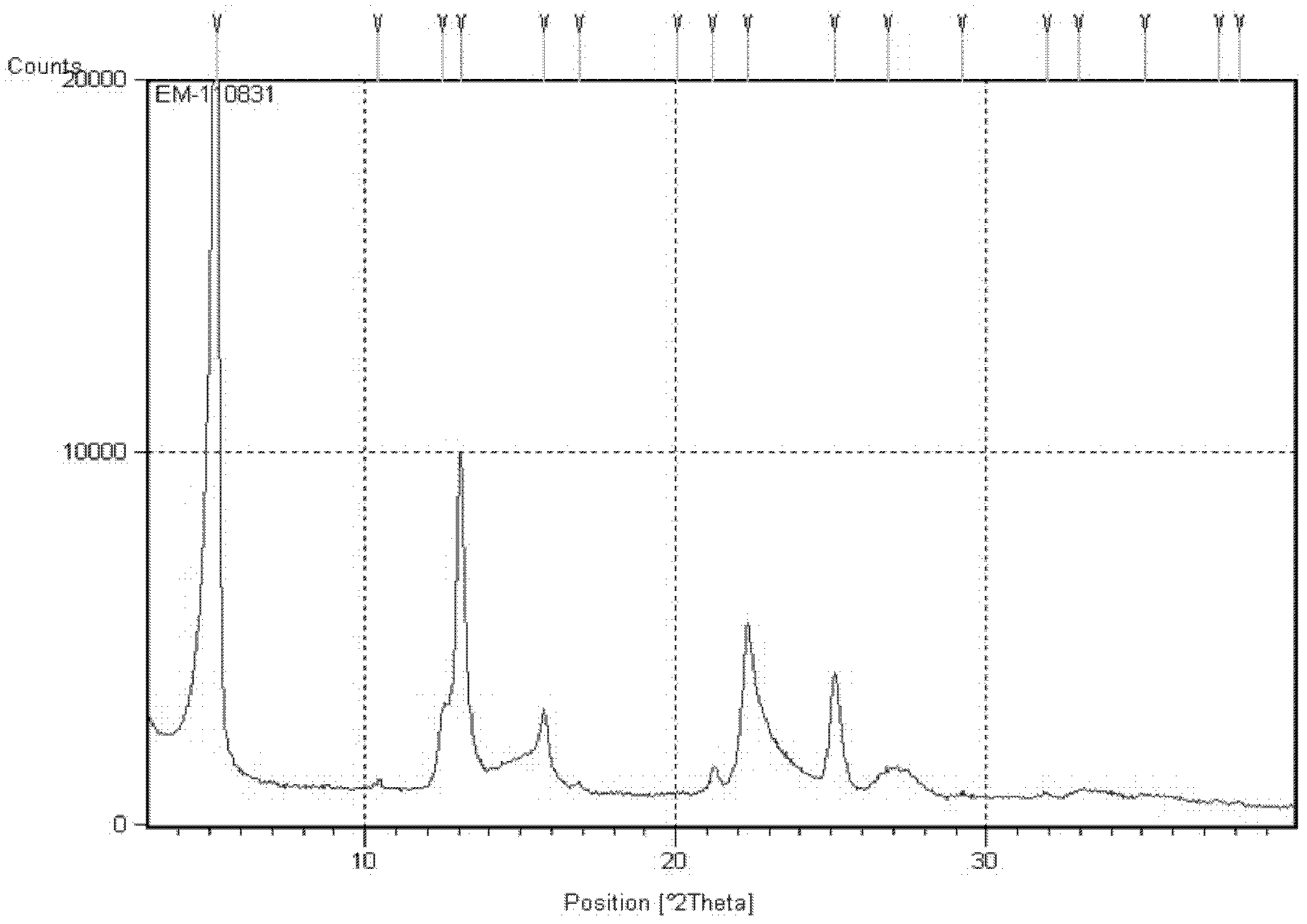
[0052] In a 1L three-necked flask, esomeprazole potassium (76.8g, absolute content 85%, 170mmol) was dissolved in deionized water (300mL), magnesium sulfate heptahydrate (49.3g, 200mmol) was dissolved in deionized water (150 mL) was slowly added dropwise to the above solution, and reacted at 35° C. for 3 hours, and a large amount of solids were precipitated. Heating was stopped, the solid was filtered out, washed with water four times, and dried under vacuum at 40°C overnight. 62.0 g of esomeprazole trihydrate was obtained with a yield of 95%. After chromatographic analysis, the HPLC purity of the product was 99.90%, and the ee value was 99.80%. X-ray powder diffraction pattern see figure 1 .
Embodiment 2
[0053] The preparation of embodiment 2 esomeprazole magnesium dihydrate type A
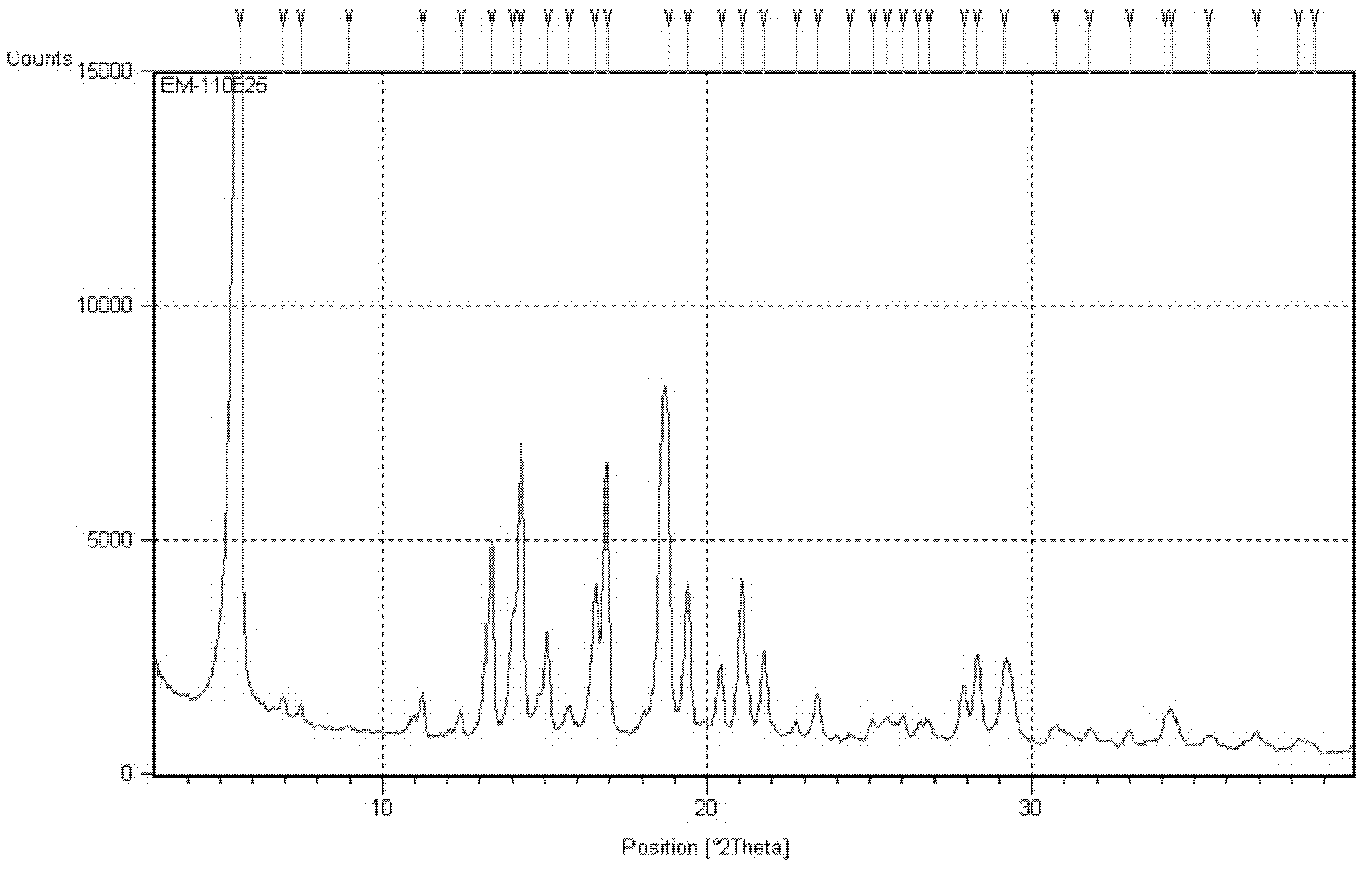
[0054] In a 250 mL flask, esomeprazole trihydrate (11.5 g, 15 mmol) was suspended in anhydrous methanol (15 mL) and stirred for 30 minutes to obtain a clear solution. Stir at 25°C for 2 hours, add a mixed solution (75mL) of acetone and water (volume ratio 4:1) at 10°C, stir overnight at this temperature, and a large amount of solids precipitate out. The solid was filtered, washed twice with acetone, and dried under vacuum at 40°C for 10 hours. Obtained 10.2 g of esomeprazole dihydrate form A with a yield of 91%. After chromatographic analysis, the HPLC purity of the product was 99.98%, and the ee value was 99.94%. X-ray powder diffraction pattern see figure 2 .
Embodiment 3
[0055] The preparation of embodiment 3 esomeprazole magnesium dihydrate type A
[0056]In a 250 mL flask, esomeprazole trihydrate (11.5 g, 15 mmol) was suspended in anhydrous methanol (15 mL) and stirred for 30 minutes to obtain a clear solution. Stir at 45°C for 1 hour, add a mixed solution (60 mL) of acetone and water (volume ratio 3:1) at 10°C, stir overnight at this temperature, and a large amount of solid precipitates out. The solid was filtered, washed twice with acetone, and dried under vacuum at 20°C for 30 hours. 10.0 g of esomeprazole dihydrate type A was obtained with a yield of 89%. After chromatographic analysis, the HPLC purity of the product was 99.98%, and the ee value was 99.92%. X-ray powder diffraction pattern see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com