Titanium oxide photocatalyst having copper compounds supported thereon, and method for producing same
一种铜化合物、光催化剂的技术,应用在化学仪器和方法、催化剂活化/制备、植物学设备和方法等方向,能够解决不能表现出催化剂活性等问题,达到光催化剂活性优异的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 manufacture example >
[0079] In this production example, to a suspension containing titanium oxide and a divalent copper compound having a rutile-type titanium oxide content of 50% by mole or more, was added to reduce divalent copper (Cu(II)) to monovalent copper (Cu(I)) reducing agent.
[0080] "Suspension preparation process"
[0081] In this step, a suspension is prepared by blending titanium oxide having a rutile-type titanium oxide content of 50 mol % or more and a divalent copper compound. After the above blending, it is preferable to prepare a suspension by stirring at, for example, 60°C or higher, preferably 70 to 100°C, more preferably 80 to 95°C for several hours, preferably about 1 hour.
[0082] (titanium oxide)
[0083] The properties of titanium oxide used in this production example (the content of rutile-type titanium oxide in the total amount of titanium oxide and the specific surface area) are the same as those described above for the copper compound-supported titanium oxide phot...
no. 2 manufacture example >
[0094] This production example includes the following steps: For a catalyst precursor containing titanium oxide having a content of rutile-type titanium oxide of 50 mol% or more and a divalent copper compound supported on the surface of the titanium oxide, nitrogen and alcohol are preferably mixed together. Light irradiation is performed in an atmosphere to reduce a part of the divalent copper compound to a monovalent copper compound.
[0095] "Catalyst Precursor"
[0096] The catalyst precursor is not particularly limited as long as it contains titanium oxide having a rutile-type titanium oxide content of 50 mol % or more and a divalent copper compound supported on the surface of the titanium oxide. For example, only a divalent copper compound may be supported on the surface of titanium oxide, or a monovalent copper compound may be supported together with a divalent copper compound. In addition, as the catalyst precursor, the existence ratio of the above-mentioned monovalent...
Embodiment 1
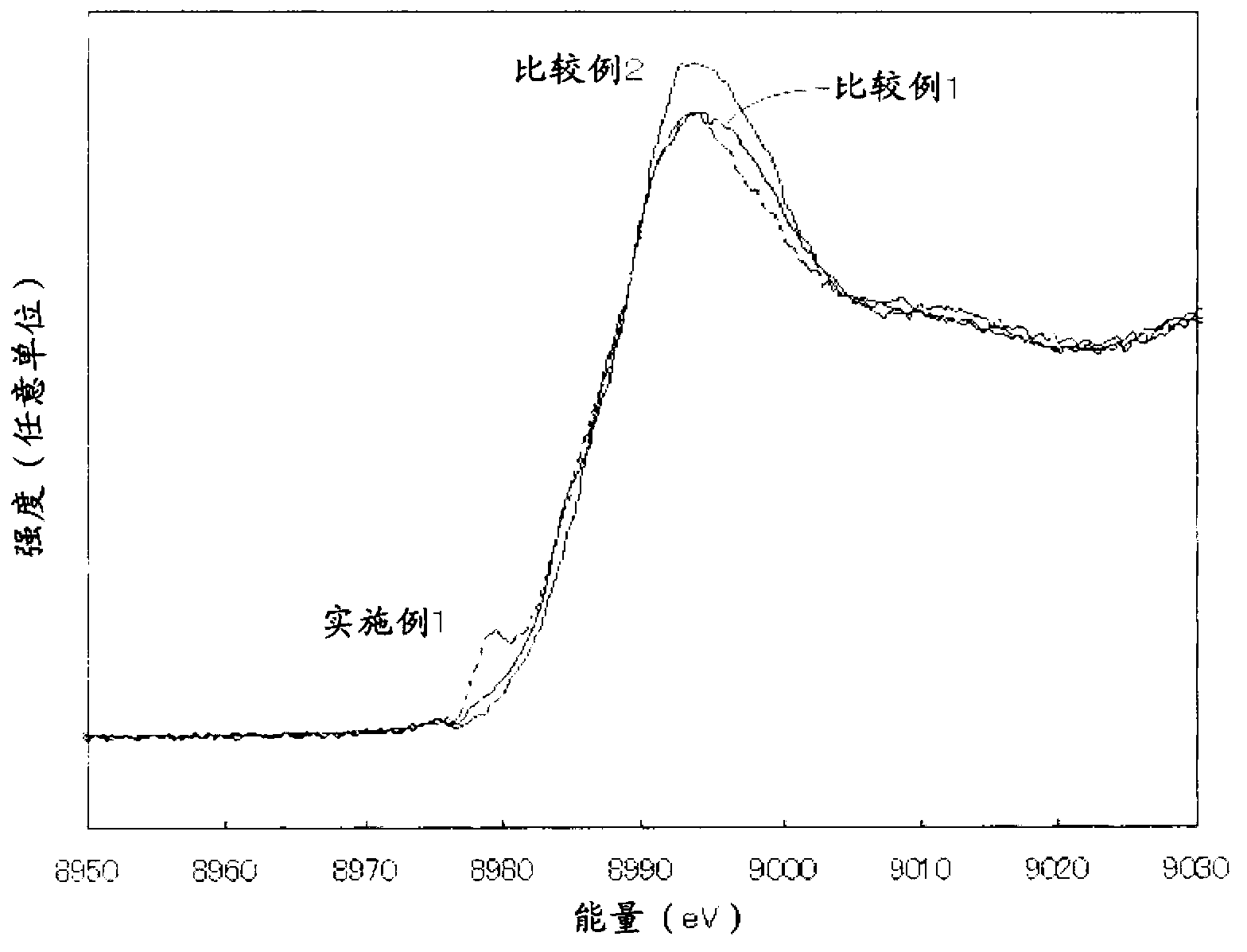
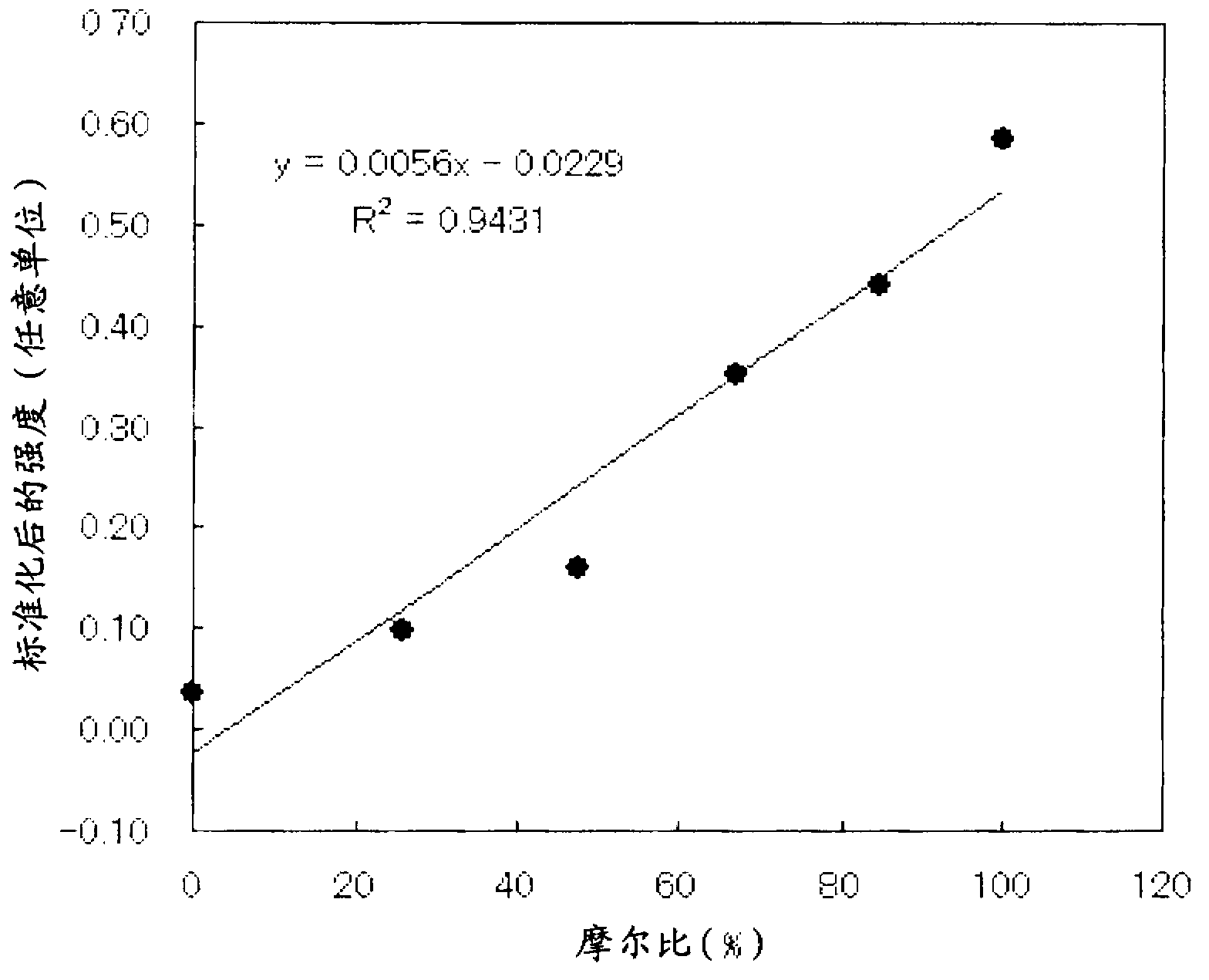
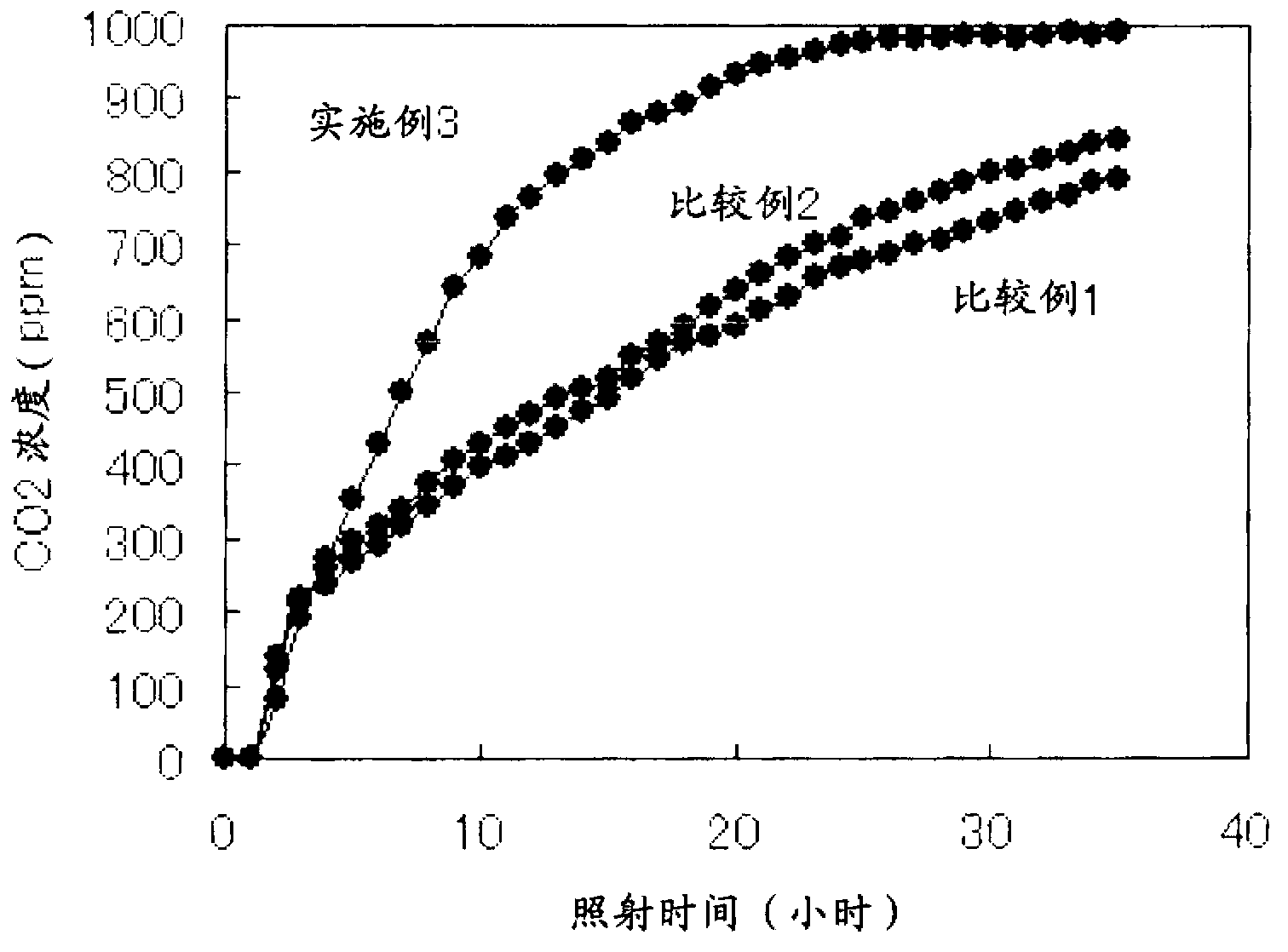
[0115] 50 g of rutile titanium oxide (F-10, manufactured by Showa Titanium Co., Ltd., BET specific surface area: 12 m 2 / g) was suspended in 1000 mL of distilled water, and 0.293 g of CuCl was added to 0.22 parts by mass of copper ions relative to 100 parts by mass of titanium oxide. 2 2H 2 O (manufactured by Kanto Chemical Co., Ltd.), heated at 90° C., and heat-treated for 1 hour while stirring. to make CuCl 2 2H 2 O:C 6 h 12 o 6 Add 1 mol / L, 13.75 ml of sodium hydroxide (manufactured by Kanto Chemical Co., Ltd.) aqueous solution and 1 mol / L, 6.88 ml of glucose (manufactured by Kanto Chemical Co., Ltd.) so that the molar ratio of NaOH is 1:4:8 Aqueous solution, heat treatment at 70°C for 1 hour. The slurry was filtered, and the obtained powder was washed with pure water, dried at 80° C., and pulverized with a mixer to obtain a sample.
[0116] The obtained sample was heated in a hydrofluoric acid solution to completely dissolve it, and the extract was quantified by IC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com