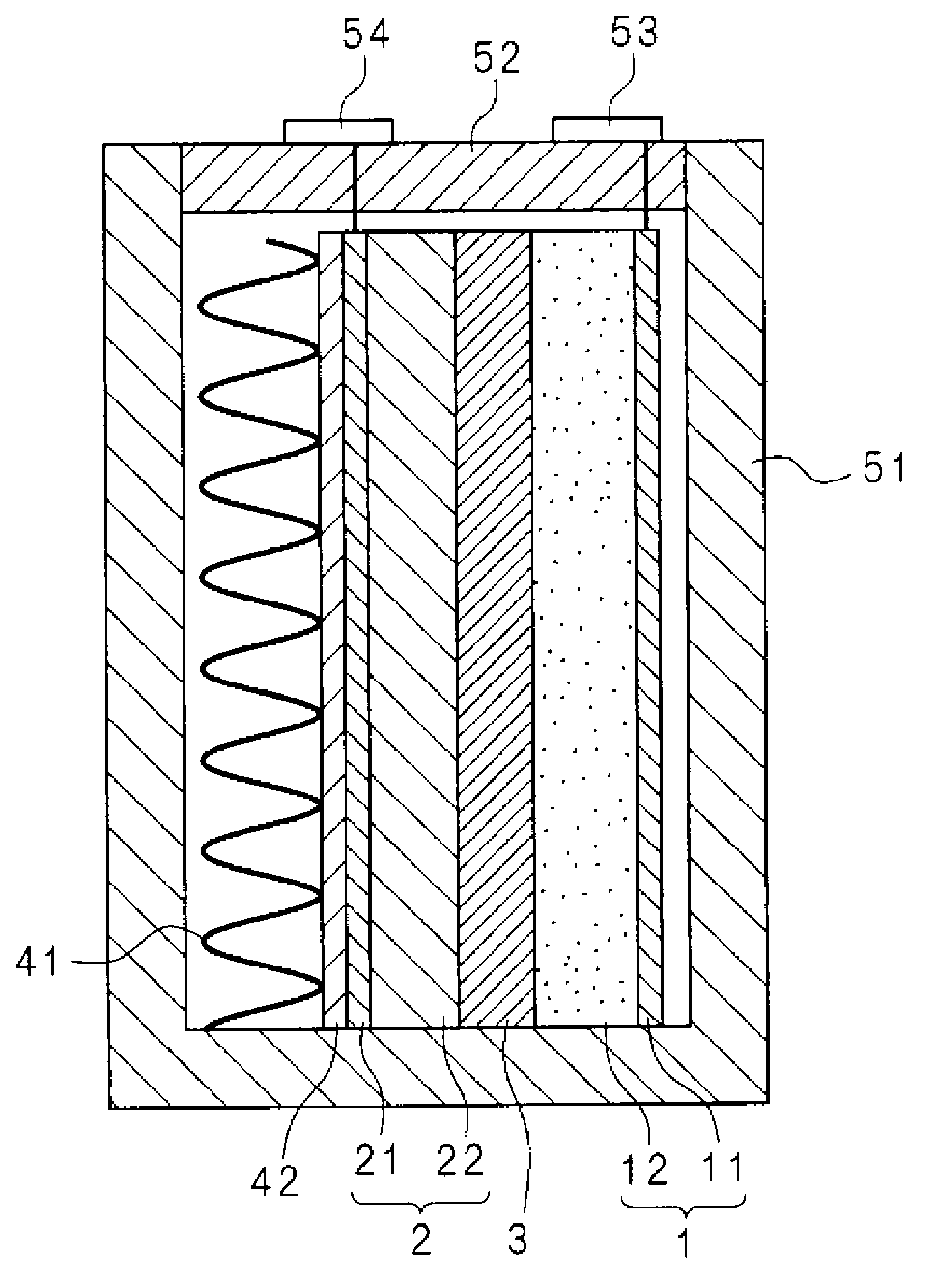
Molten salt battery
A molten salt battery, molten salt technology, applied in molten electrolytes, battery electrodes, secondary batteries, etc., can solve problems such as increasing the cost, scarcity, and deterioration of molten salt batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
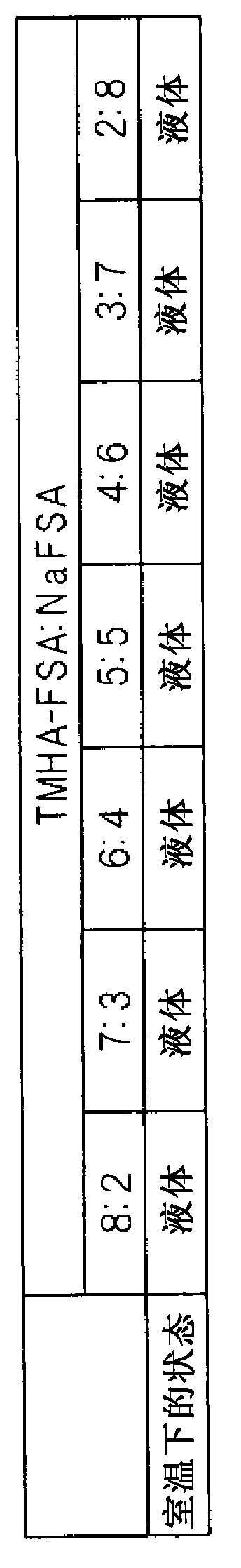
[0098] As a molten salt, a mixed salt of TMHA-FSA and NaFSA was prepared. Then, the relationship between the state of the mixed salt at room temperature and the molar ratio of TMHA-FSA to NaFSA in the mixed salt was studied. First, TMHA-Br manufactured by Wako Pure Chemical Industries, Ltd. and KFSA manufactured by Mitsubishi Materials Corporation were mixed in water at an equimolar ratio to prepare TMHA-FSA. Then, the resulting precipitate was filtered and washed several times with water. Subsequently, TMHA-FSA was prepared by performing vacuum drying at 80°C. Note that Br is bromine and K is potassium. The prepared TMHA-FSA and NaFSA manufactured by Mitsubishi Materials Corporation, Ltd. were mixed at various molar ratios in a glove box under an argon atmosphere to study their melting behavior at room temperature.
[0099] figure 2 It is a table showing the molar ratio in the mixed salt of TMHA-FSA and NaFSA and the state of the mixed salt at each molar ratio at room te...
Embodiment 2
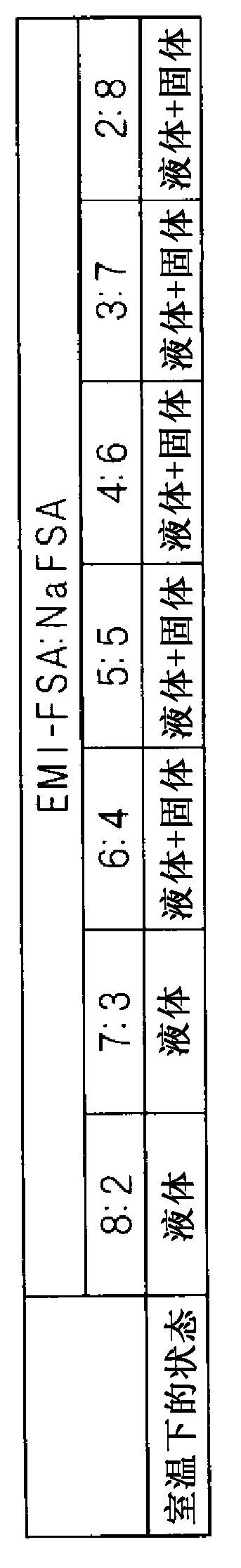
[0101] As a molten salt, a mixed salt of EMI-FSA and NaFSA was prepared. Then, the relationship between the state of the mixed salt at room temperature and the molar ratio of EMI-FSA to NaFSA in the mixed salt was studied. EMI-FSA was obtained from Tokyo Chemical Industry Co., Ltd. EMI-FSA and NaFSA manufactured by Mitsubishi Materials Corporation, Ltd. were mixed at various molar ratios in a glove box under an argon atmosphere to study their melting behavior at room temperature.
[0102] image 3 It is a table showing the molar ratio in the mixed salt of EMI-FSA and NaFSA and the state of the mixed salt at each molar ratio at room temperature. Such as image 3 The molar ratios of EMI-FSA to NaFSA (EMI-FSA:NaFSA) were prepared as shown in 8:2, 7:3, 6:4, 5:5, 4:6, 3:7 and 2:8, respectively. of seven mixed salts. Mixed salts with molar ratios of 8:2 and 7:3 are liquid at room temperature. In addition, mixed salts of other molar ratios are in a mixed state of liquid and sol...
Embodiment 3
[0104] As a molten salt, a mixed salt of P13-FSA and NaFSA was prepared. Then, the relationship between the state of the mixed salt at room temperature and the molar ratio of P13-FSA to NaFSA in the mixed salt was studied. P13-FSA was obtained from Tokyo Chemical Industry Co., Ltd. P13-FSA and NaFSA manufactured by Mitsubishi Materials Corporation, Ltd. were mixed at various molar ratios in a glove box under an argon atmosphere to study their melting behavior at room temperature.
[0105] Figure 4 It is a table showing the molar ratio in the mixed salt of P13-FSA and NaFSA and the state of the mixed salt at each molar ratio at room temperature. Such as Figure 4 As shown in , the molar ratios of P13-FSA to NaFSA (P13-FSA:NaFSA) were prepared as 8:2, 7:3, 6:4, 5:5, 4:6, 3:7 and 2:8 of seven mixed salts. Various mixed salts with molar ratios of 8:2, 7:3, 6:4, 5:5 and 4:6 are liquid at room temperature. In addition, molten salts of other molar ratios are in a mixed state o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com