In-situ gel for injecting flunixin meglumine and preparation method thereof
A technology of flunixin meglumine and in-situ gel, which is applied in the field of in-situ gel for flunixin meglumine injection and its preparation, can solve the problems of time-consuming animals, stress, etc., and achieve convenient administration , good physiological compatibility, low irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
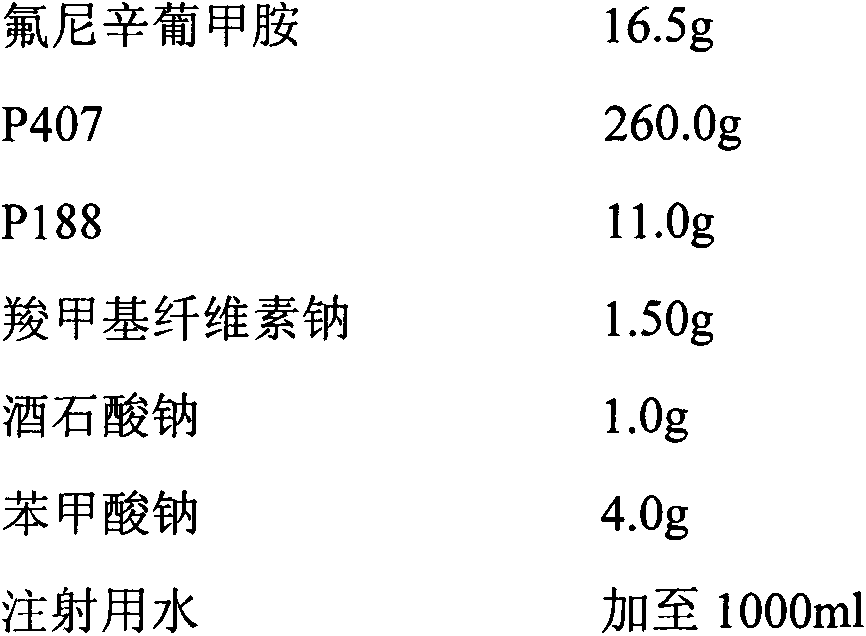
[0025] 1% flunixin meglumine in situ gel for injection (calculated as flunixin)
[0026] prescription
[0027]
[0028] Preparation method: Take an appropriate amount of water for injection, add the prescribed amount of flunixin meglumine, and stir to dissolve; dissolve sodium tartrate and sodium benzoate with a small amount of water for injection respectively, and add them to the above-mentioned flunixin meglumine solution; Sprinkle the gel matrix and sodium carboxymethyl cellulose on the liquid surface, refrigerate at about 4°C until a clear, lump-free solution is obtained, filter through a 0.22 μm microporous membrane; dilute to volume with water for injection, and stir well , that is.
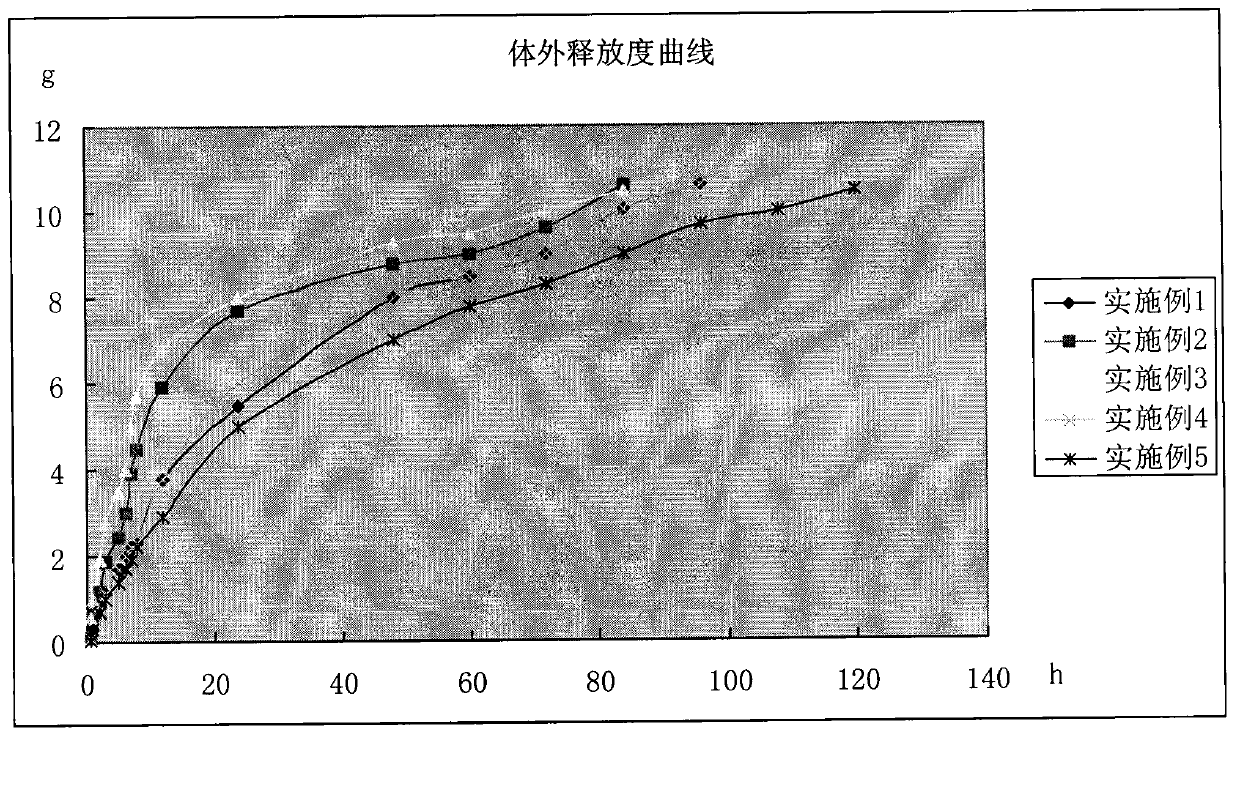
[0029] The prepared in situ gel was evaluated in vitro, including gelation temperature, gelation time, thermal reversibility, release rate, viscosity, pH value, etc. The measurement methods of each item are as follows, the measurement results are shown in Table 1, and the in vitro rele...
Embodiment 2
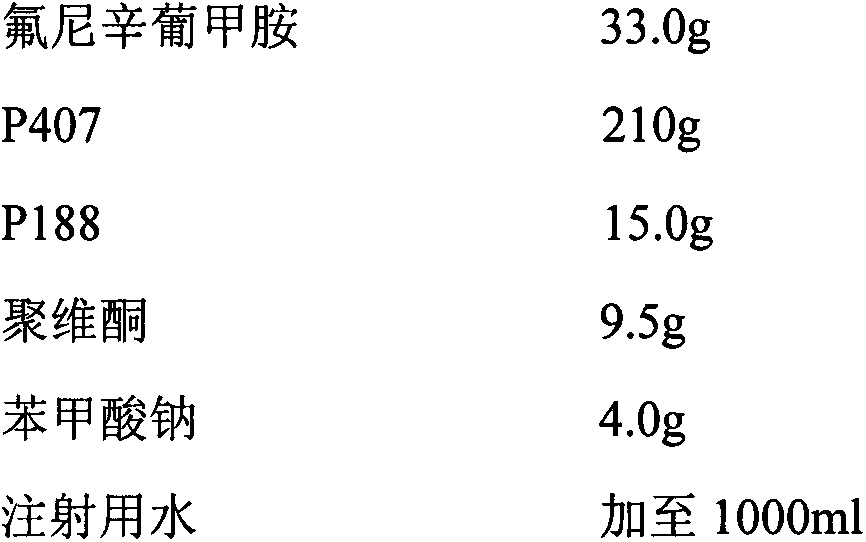
[0037] 2% flunixin meglumine in situ gel for injection (calculated as flunixin)
[0038] prescription
[0039]
[0040] Preparation method: Take an appropriate amount of water for injection, add the prescribed amount of flunixin meglumine, and stir to dissolve; dissolve sodium benzoate with a small amount of water for injection, and add it to the above-mentioned flunixin meglumine solution; then add the prescribed amount of gel matrix Sprinkle it with povidone on the liquid surface, refrigerate at about 4°C until a clear, lump-free solution is obtained, filter through a 0.22μm microporous membrane; dilute with water for injection, stir evenly, and obtain. The in vitro performance evaluation method is the same as in Implementation 1, and the results are shown in Table 1, and the in vitro release results are shown in the accompanying drawings.
Embodiment 3
[0042] 5% flunixin meglumine in situ gel for injection (calculated as flunixin)
[0043] prescription
[0044]
[0045]
[0046] Preparation method: Take an appropriate amount of water for injection, add the prescribed amount of flunixin meglumine, and stir to dissolve; soak the carbomer with an appropriate amount of water for injection, adjust to dissolve with benzalkonium bromide and sodium hydroxide aqueous solution, and add to the above Flunixin meglumine solution; then sprinkle the prescribed amount of gel matrix on the liquid surface, refrigerate at about 4°C until a clear, lump-free solution is obtained, filter through a 0.22 μm microporous membrane; use water for injection After constant volume, stir well, that is. First, other operations and in vitro performance evaluation methods are the same as in implementation 1. The results are shown in Table 1, and the in vitro release results are shown in the accompanying drawings.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com