Zedoary turmeric lactone enantiomer and preparation method thereof
A technology of enantiomer and curcumolactone, which is applied in the field of enantiomer of curcumolactone and its preparation, to achieve good anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
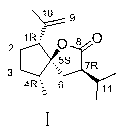
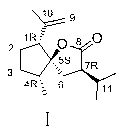
[0038] 1. Preparation of 8-OH-Neocrudione (Ⅵ)
[0039] Add Neocurdione 500mg (2.12mmol) to a 50ml round-bottomed flask with a stirring bar, dissolve it in 6ml of methanol, and add NaBH in batches under ice-cooling 4 100mg (2.64mmol), stirred reaction at 0°C. After reacting for 4.5 hours, the reaction solution was evaporated to dryness, 3ml of water was added, adjusted to neutrality with 1N HCl, extracted three times with an equal volume of ethyl acetate, and the ester layer was evaporated to dryness to obtain oily product VI. It does not need to be purified and can be directly used for the next reaction.
[0040] 2. 8-OH-4- epi - Preparation of neocurdione (VII)
[0041] with CaCl 2Add dry oily product VI to a 50ml round bottom flask with a drying tube, a spherical condenser, and a stirring bar, dissolve it in 10ml of anhydrous benzene, add 60mg (0.53mmol) of potassium tert-butoxide, and reflux at 83°C for 6h until the reaction reaches equilibrium . The product was sep...
Embodiment 2
[0047] 1. Preparation of 8-OH-Neocrudione (Ⅵ)
[0048] Add Neocurdione 500mg (2.12mmol) to a 50ml round bottom flask with a stirring bar, dissolve it in 15ml methanol, add NaBH in batches under ice-cooling 4 100mg (2.64mmol), heated to 50°C and stirred to react. After reacting for 2 hours, the reaction solution was evaporated to dryness, 3ml of water was added, adjusted to neutrality with 1N HCl, extracted three times with an equal volume of ethyl acetate, and the ester layer was evaporated to dryness to obtain oily product VI. It does not need to be purified and can be directly used for the next reaction.
[0049] 2. 8-OH-4- epi - Preparation of neocurdione (VII)
[0050] with CaCl 2 Add dry oily product VI to a 50ml round bottom flask with a drying tube, a spherical condenser, and a stirrer, dissolve it in 10ml of anhydrous benzene, add 60mg (0.53mmol) of potassium tert-butoxide, and react at room temperature for 6h until the reaction reaches equilibrium. The product ...
Embodiment 3
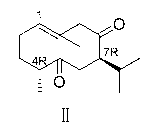
[0052] Preparation of (-)-Curdione (Ⅱ)
[0053] Synthesize compound VII8-OH-4- with the method in embodiment 1 or 2 epi -neocurdione, add 72.3mg (0.30mmol) of compound VII to a 50ml round bottom flask with a stirring bar, add 5ml of dichloromethane as a solvent, add 225.7mg (0.6mmol) of PDC, and stir the reaction at room temperature for 2h. Add 0.5 g of silica gel to the reaction solution, continue stirring for 0.5 h, filter, and evaporate to dryness to obtain 69.5 g of white solid II, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com