Liquid preparation for oral administration used in CT colonography, and composition for imaging digestive tract
A technology for the digestive tract and compounds, which is used in drug delivery, preparation of X-ray contrast agents, preparations for in vivo experiments, etc. Ease of use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
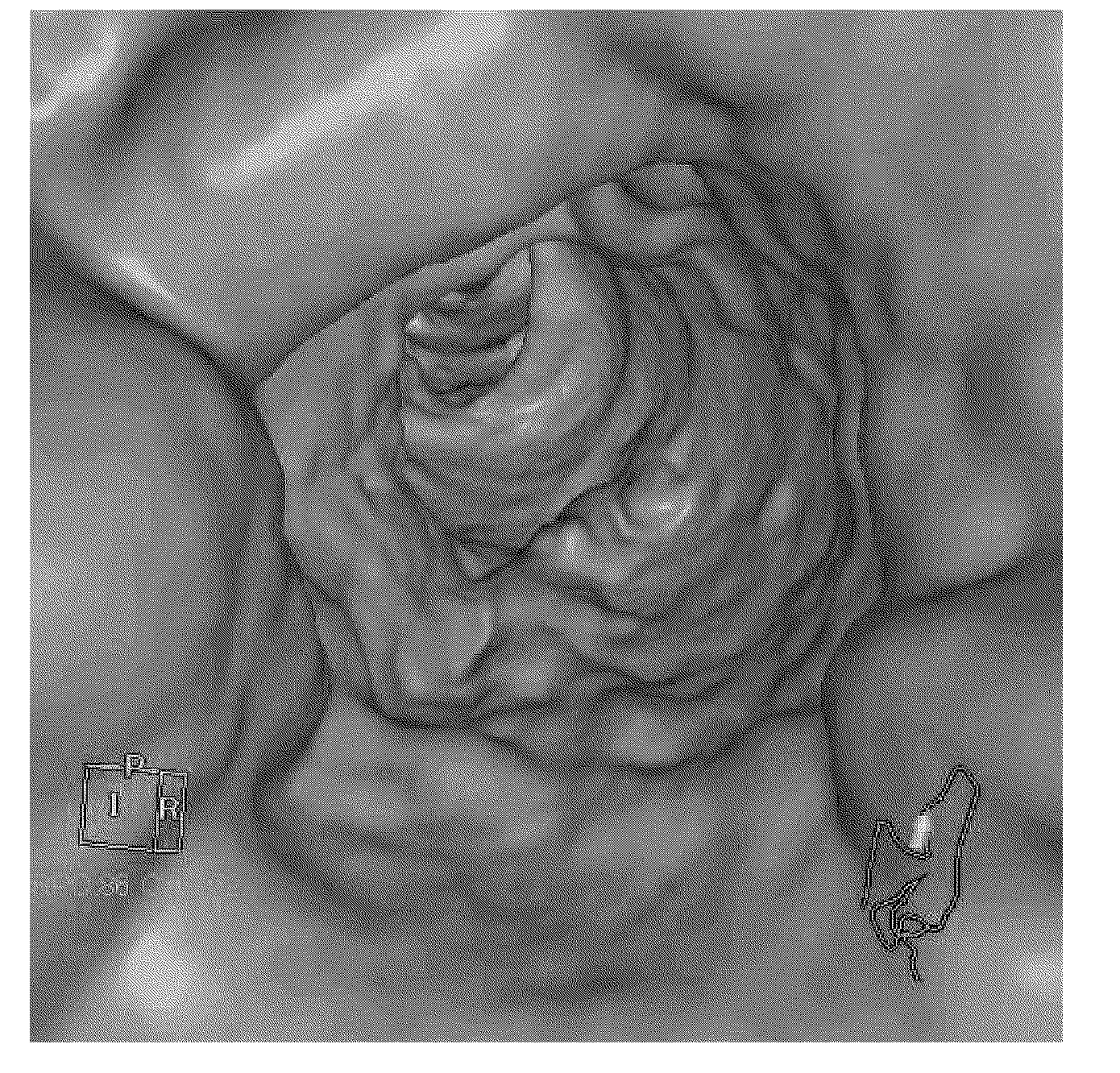
[0127] On the day before CT colonography examination, for the situation of taking 800mL oral administration liquid of the present invention (A group, 2 people), taking 800mL does not contain water-soluble macromolecule and does not contain salt cathartic (does not contain enough The case of an iodine-containing compound solution (group B, 2 persons), the case of taking 400 mL (half the amount of group A) of the same solution as that of group A (group C, 3 persons), When 40 mg of mosapride citrate (group D, 1 person) was administered in addition to the same liquid preparation as group A in the same volume as group A, colon images obtained by CT imaging were evaluated.
[0128] The specific pretreatment of each group is as follows. It should be noted that the iodine-based water-soluble contrast medium uses the trade names "Omnipaque (registered trademark) Note 350 (manufactured by Daiichi Sankyo Pharmaceutical Co., Ltd.)" (generic name: iohexol), "Ioverin (registered trademark) ...
Embodiment 2
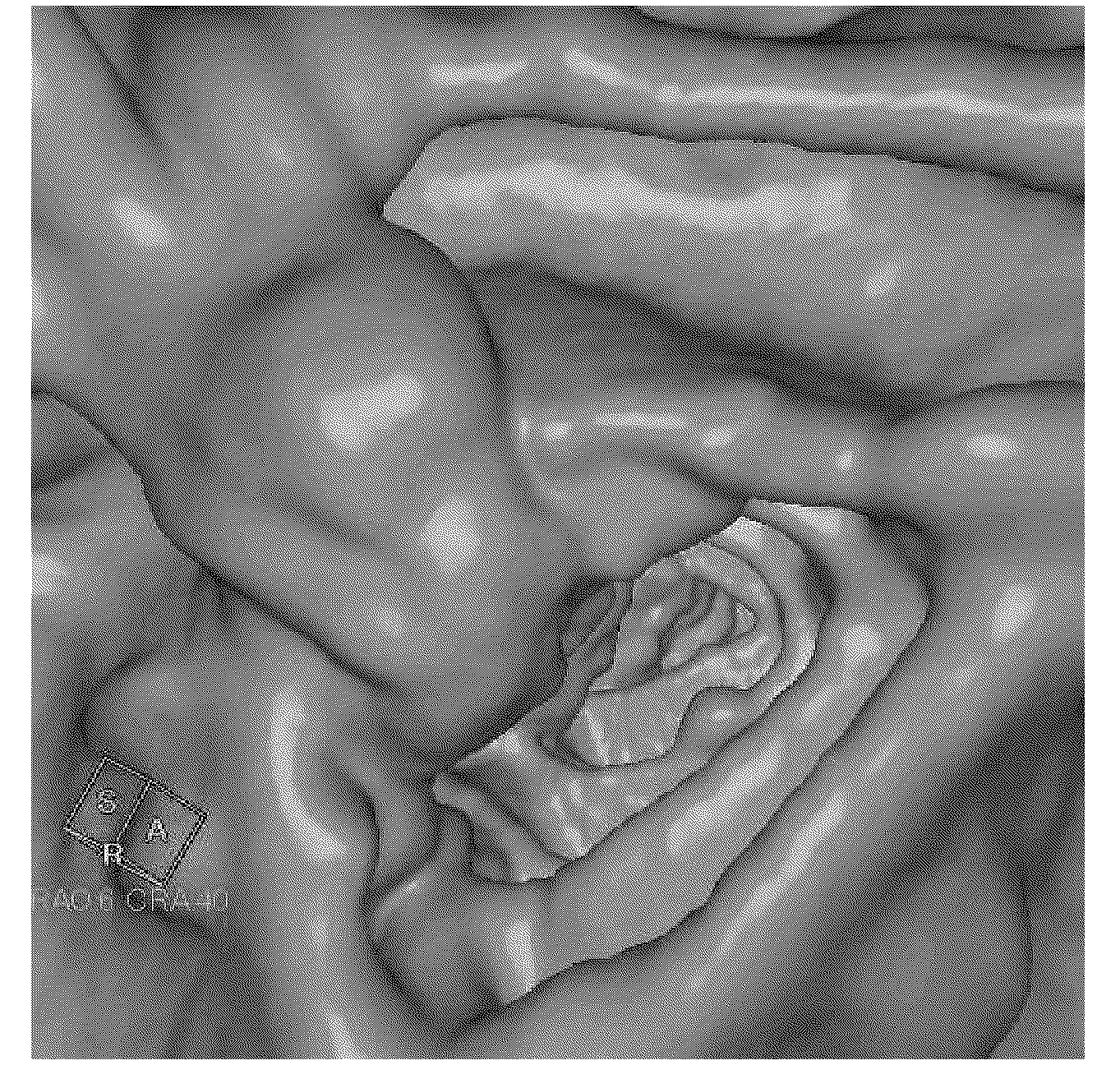
[0146] The images of the large intestine obtained by CT colonography were evaluated for groups A to G (two persons in each group) who took 300 to 450 mL of the liquid preparation for oral administration of the present invention on the day before the CT colonography examination. Cases in all groups took 20 mg of mosapride citrate (trade name "Gasmotin (registered trademark) tablets", manufactured by Dainippon Sumitomo Pharmaceutical Co., Ltd.) twice after breakfast and dinner on the day before the examination, and started MDCT after dinner. Fasting until the end of imaging, only water intake. In addition, 2 tablets of sodium picosulfate (trade name "Shinluck (registered trademark) tablet 2.5" manufactured by Iwaki Pharmaceutical Co., Ltd.) (5 mg as sodium picosulfate hydrate) were taken orally after dinner on the day before the examination. However, Case 5 of Group C did not take either mosapride citrate or sodium picosulfate orally.
[0147] The specific pretreatment of each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com