Cefdinir, citric acid and sodium citrate dry suspension composition
A technology of cefdinir and sodium citrate, which is applied in the direction of medical preparations of non-active ingredients, organic active ingredients, antibacterial drugs, etc., which can solve the inconvenience of patients taking, the bioavailability and stability of cefdinir drugs Low-level problems, to achieve the effect of easy portability, improved bioavailability and stability, and suitable for large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
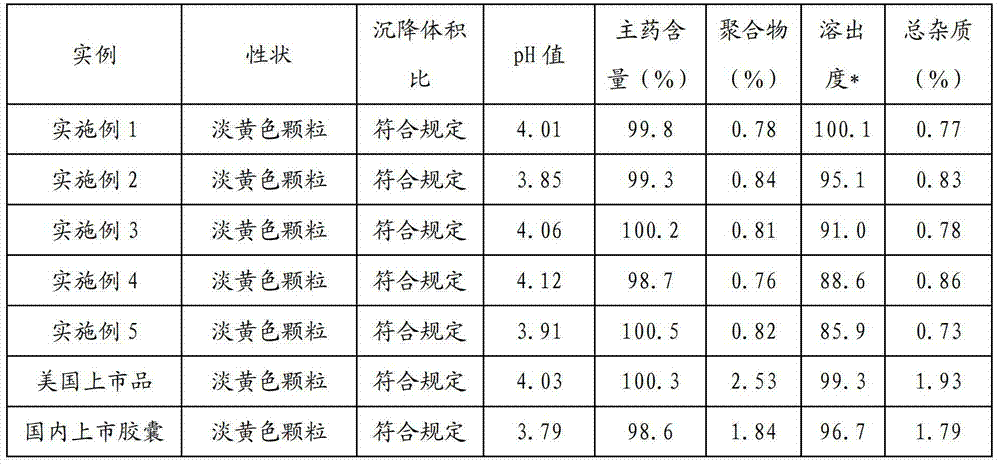
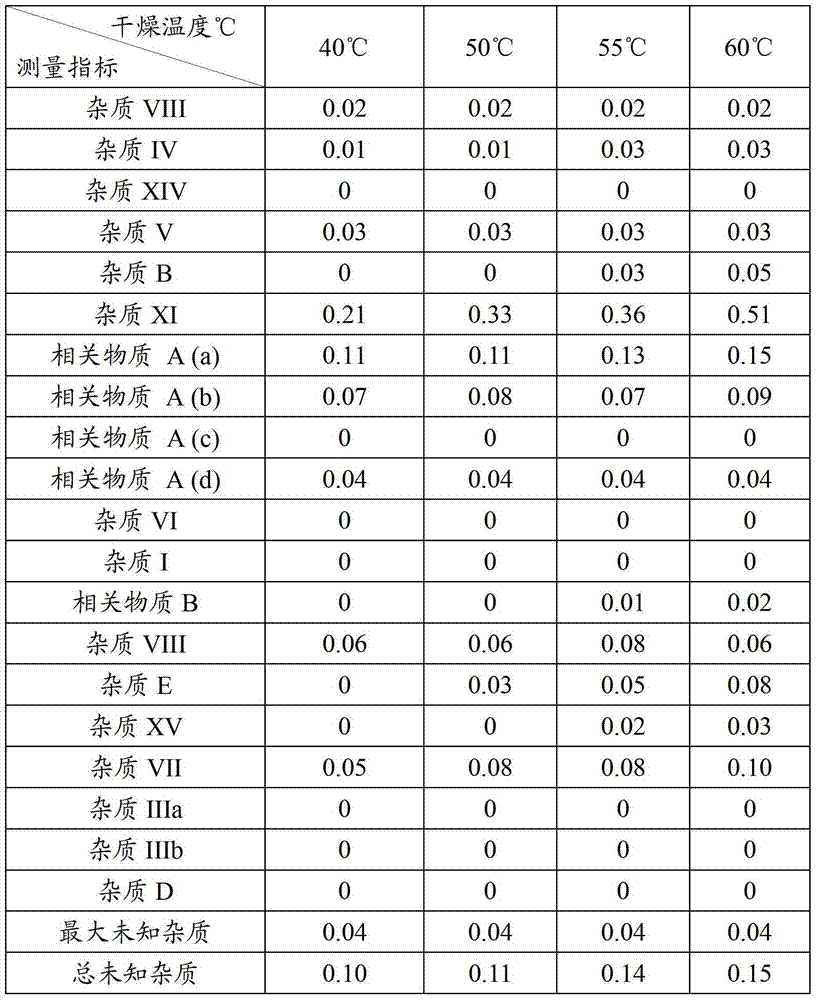
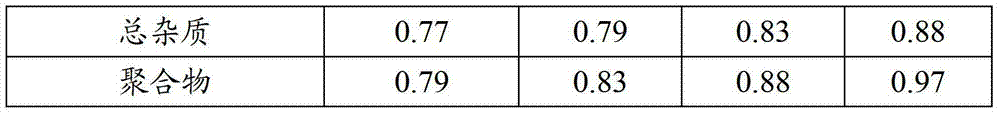
Examples
preparation Embodiment 1
[0042] Prepare the cefdinir dry suspension of the present embodiment according to the following composition ratio:
[0043] Cefdinir 100g Sucrose 2000g
[0044] Sodium citrate 80g Citric acid 80g
[0045] Strawberry powder flavor 32g 50% (wt) ethanol aqueous solution 190g
[0046] Xanthan gum 20g Micronized silica gel 10g
[0048] Step 1, pulverizing sucrose, citric acid and sodium citrate;
[0049] Step 2, passing cefdinir through a 200-mesh sieve, passing sucrose, citric acid, sodium citrate and magnesium stearate through a 100-mesh sieve, passing strawberry powder essence, xanthan gum and micronized silica gel through a 80-mesh sieve;
[0050] Step 3, weigh each component according to the above content, first add sucrose and citric acid into the high-speed mixing granulator and mix for 1min, the mixing speed is 400rpm, and the cutting speed is 3000rpm, then sodium citrate, strawberry powder essence and Add cefdinir, turn on the granulatin...
preparation Embodiment 2
[0054] Prepare the cefdinir dry suspension of the present embodiment according to the following composition ratio:
[0055] Cefdinir 100g Sucrose 2100g
[0056] Sodium citrate 60g Citric acid 60g
[0057] Strawberry powder essence 35g 50% (wt) ethanol aqueous solution 185g
[0058] Xanthan gum 30g Micronized silica gel 8g
[0059] Magnesium Stearate 1.5g
[0060] Step 1, pulverizing sucrose, citric acid and sodium citrate;
[0061] Step 2, passing cefdinir through a 200-mesh sieve, passing sucrose, citric acid, sodium citrate and magnesium stearate through a 100-mesh sieve, passing strawberry powder essence, xanthan gum and micronized silica gel through a 80-mesh sieve;
[0062] Step 3, weigh each component according to the above content, first add sucrose and citric acid into the high-speed mixing granulator and mix for 1min, the mixing speed is 400rpm, and the cutting speed is 3000rpm, then sodium citrate, strawberry powder essence and Add cefdinir, turn on the granulat...
preparation Embodiment 3
[0066] Prepare the cefdinir dry suspension of the present embodiment according to the following composition ratio:
[0067] Cefdinir 100g Sucrose 2100g
[0068] Sodium citrate 90g Citric acid 90g
[0069] Strawberry powder flavor 28g 50% (wt) ethanol aqueous solution 190g
[0070] Xanthan gum 20g Micronized silica gel 10g
[0072] Step 1, pulverizing sucrose, citric acid and sodium citrate;
[0073] Step 2, passing cefdinir through a 200-mesh sieve, passing sucrose, citric acid, sodium citrate and magnesium stearate through a 100-mesh sieve, passing strawberry powder essence, xanthan gum and micronized silica gel through a 80-mesh sieve;
[0074] Step 3, weigh each component according to the above content, first add sucrose and citric acid into the high-speed mixing granulator and mix for 1min, the mixing speed is 400rpm, and the cutting speed is 3000rpm, then sodium citrate, strawberry powder essence and Add cefdinir, turn on the granulatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com