Method for preparing theophylline sustained release
A technology for sustained-release tablets and theophylline, which is applied in the field of medicine, can solve the problems of poor reproducibility of sustained-release tablets, differences in release rate and dissolution, and achieve the effects of improving use value, stable quality, and avoiding toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
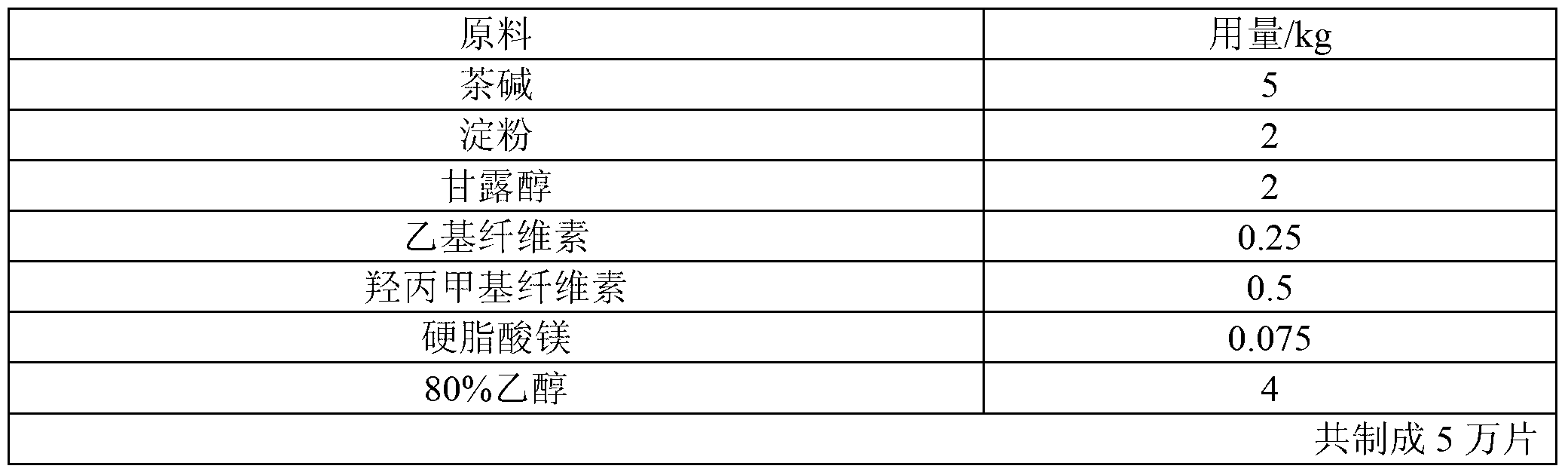
[0033] Table 1 The raw materials and proportions of theophylline sustained-release tablets (100mg)
[0034]
[0035] The preparation method is as follows:
[0036] (1) Add ethyl cellulose, hydroxypropyl methyl cellulose and 80% ethanol of recipe quantity in the wet mixing granulator, stir and shear at normal temperature for 30 minutes, and make a uniformly dispersed binder for subsequent use;
[0037] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0038] (3) After replacing the granulator with a 16-mesh stainless steel mesh, use the granulator to granulate;
[0039] (4) The prepared wet granules are pumped into the fluidized bed, and the wet granules are vacuum pumped into the fluidized bed to dry to a moisture content of 4%, and then the granules are sized with a crushing and sizing machine, and magnesium stearate is added to mix evenly and then pressed The tablets are dried to obtain theophylline su...
Embodiment 2
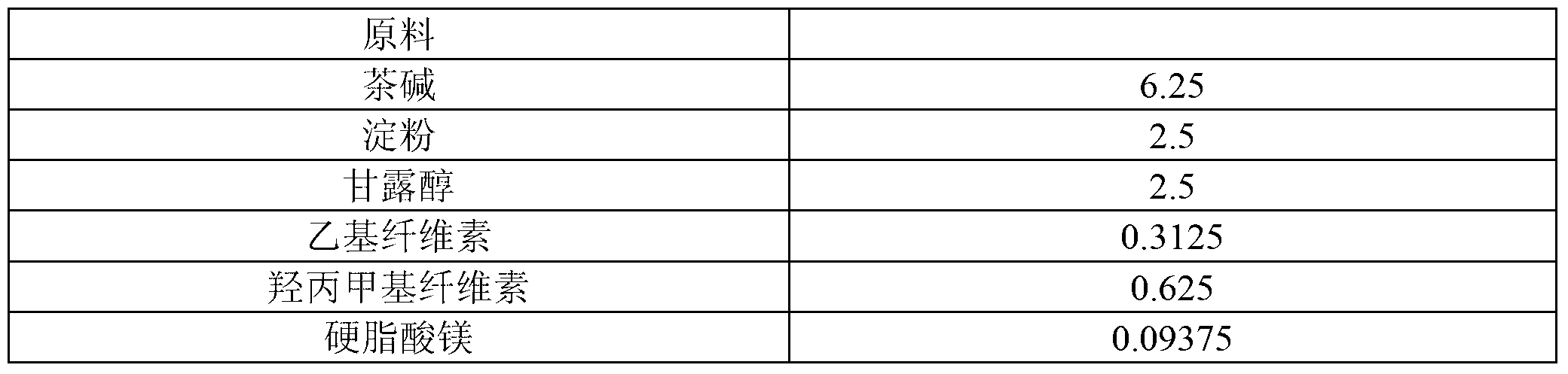
[0041] Table 2 Theophylline Sustained-release Tablets (125mg) Raw Materials and Proportioning
[0042]
[0043]
[0044] The preparation method is as follows:
[0045] (1) Add povidone K30, methyl cellulose and 80% ethanol of recipe quantity in the wet mixing granulator, stir and shear at normal temperature for 25 minutes, make the uniformly dispersed adhesive for subsequent use;
[0046] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0047] (3) After replacing the granulator with 18-mesh stainless steel mesh, use the granulator to granulate;
[0048] (4) The prepared wet granules are pumped into the fluidized bed, and the wet granules are vacuum pumped into the fluidized bed to dry to 5% moisture, and then the granules are sized with a crushing and sizing machine, and magnesium stearate is added to mix evenly and then pressed The tablets are dried to obtain theophylline sustained-release tablets. ...
Embodiment 3
[0050] Table 3 The raw materials and proportions of theophylline sustained-release tablets (200mg)
[0051] raw material
Dosage / kg
5
4
0.25
carbomer
0.1
0.5
0.01
0.075
80% ethanol
4
A total of 25,000 pieces were made
[0052] The preparation method is as follows:
[0053] (1) Add sodium alginate, hydroxypropyl methylcellulose and 80% ethanol in the wet mixing granulator, stir and shear at room temperature for 30 minutes, and make a uniformly dispersed adhesive for subsequent use;
[0054] (2) Add theophylline, binding agent, starch and mannitol of recipe quantity in granulator again, make soft material;
[0055] (3) After replacing the granulator with a 19-mesh stainless steel mesh, use the granulator to granulate;
[0056] (4) The prepared wet granules are pumped into the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com