Preparation method of polyferric sulfate (PFS)
A technology for polymerizing ferric sulfate and persulfate, which is applied in the fields of ferric sulfate, flocculation/precipitation water/sewage treatment, etc., can solve the problems of increased process cost, waste of oxidant, complicated equipment structure, etc., and achieves easy control of the injection speed and suitable pH. Wide range of effects with simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
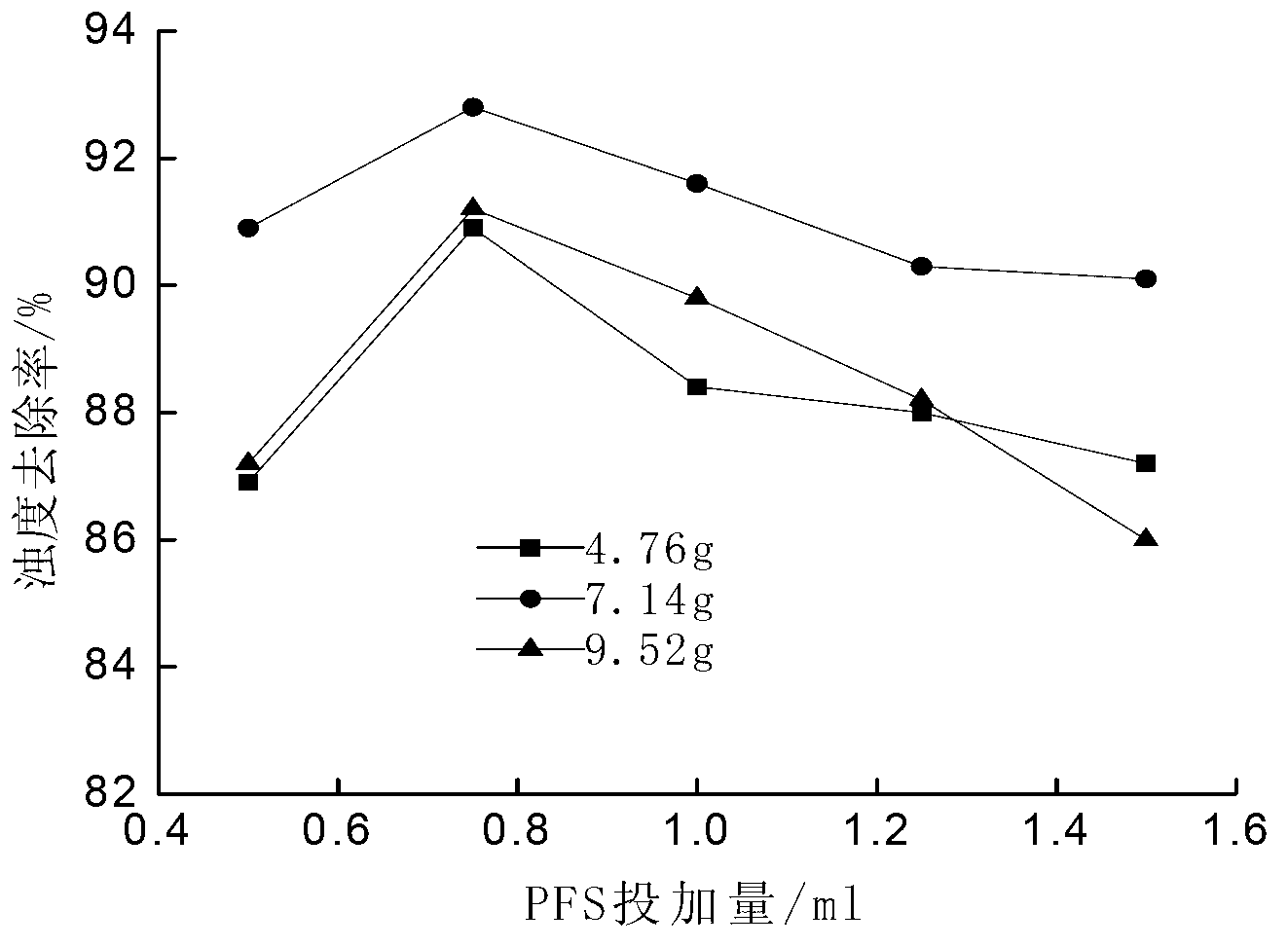
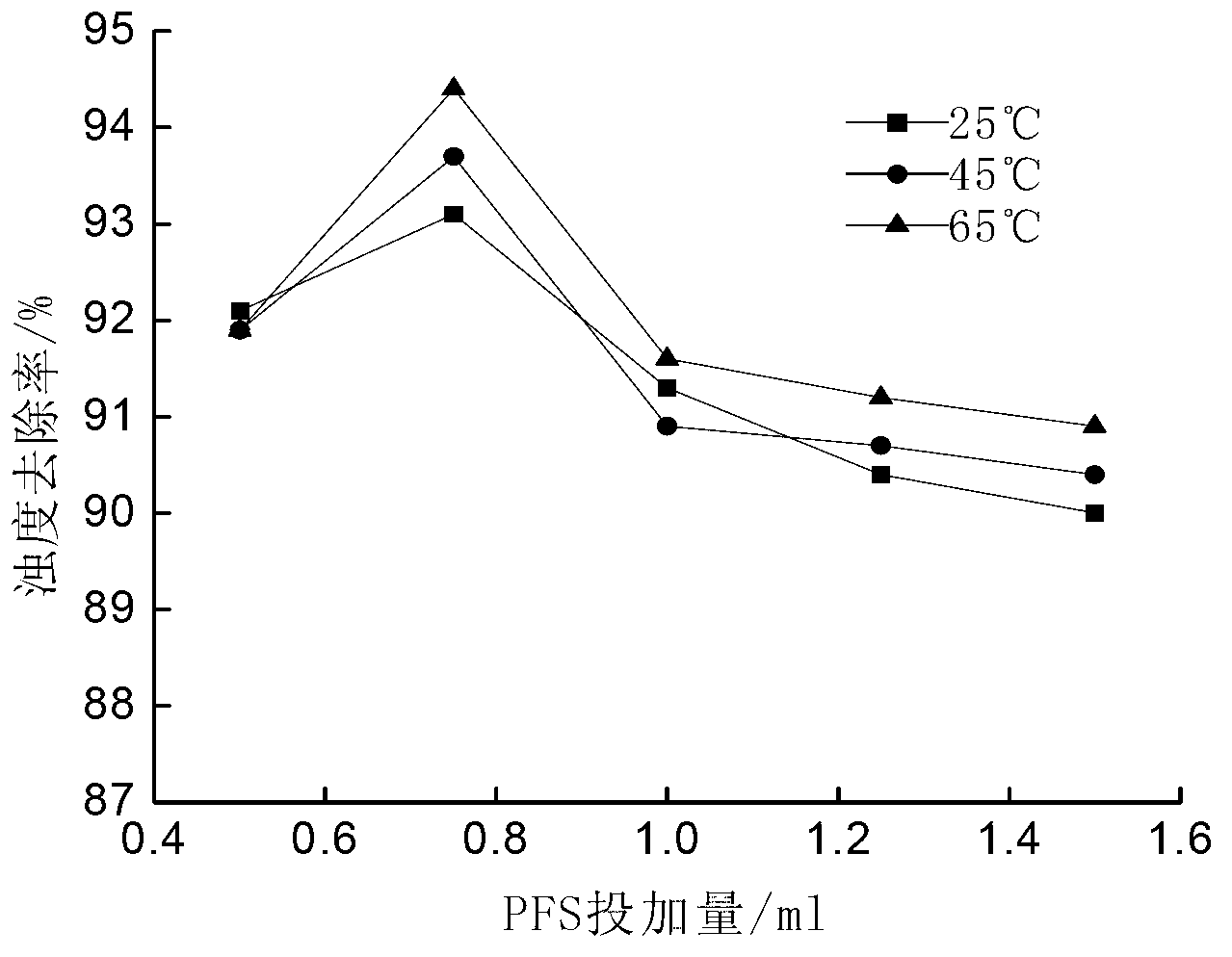
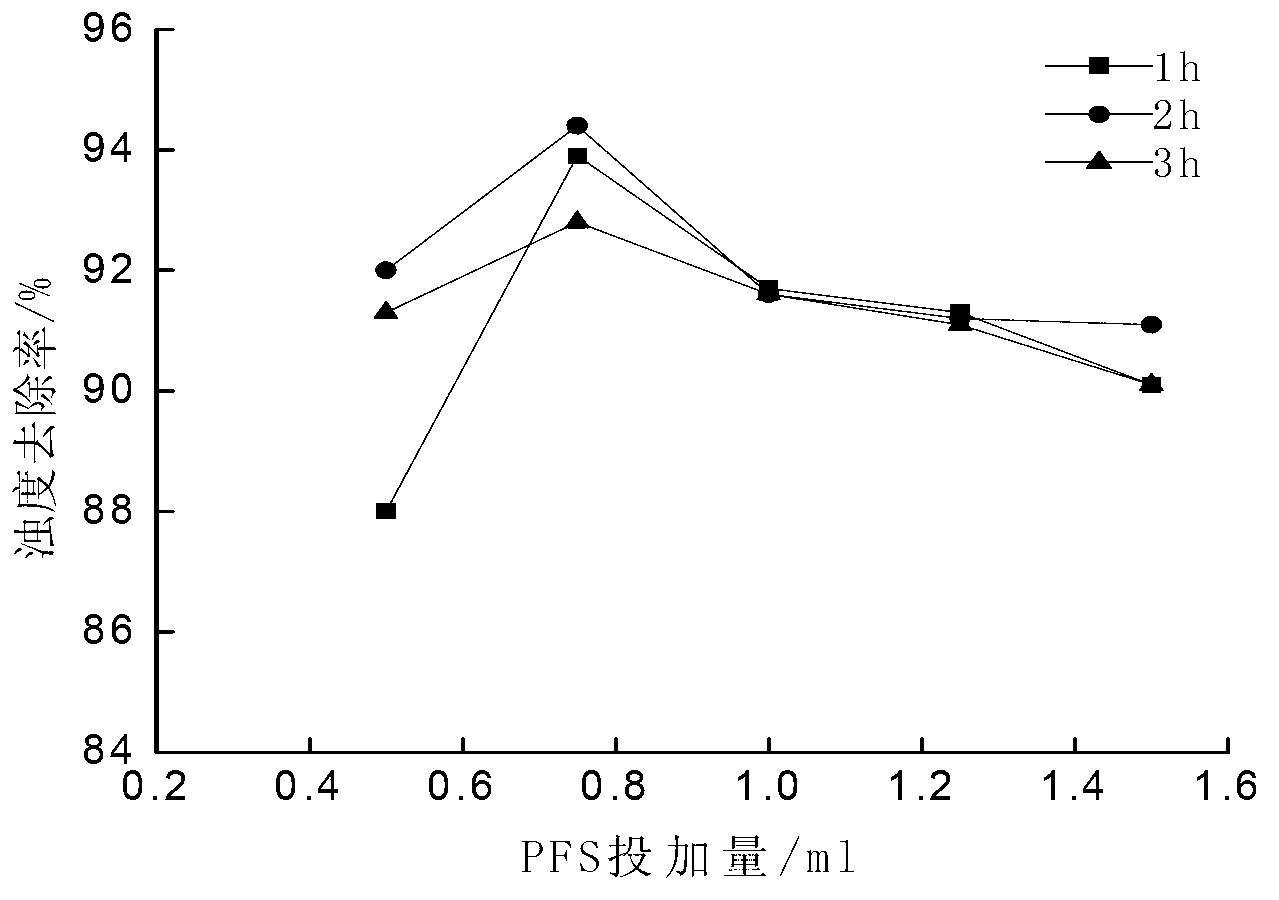
[0016] Taking ferrous sulfate as a soluble ferrous salt as an example, the method for preparing polyferric sulfate is: weigh 13.9g FeSO 4 Placed in a 250mL Erlenmeyer flask, add 50mL of distilled water to FeSO 4 The concentration is 56g / L, add 7.14 g Na 2 S 2 o 8 , FeSO 4 with Na 2 S 2 o 8 The molar ratio of 1:0.6 ensures that Fe in the solution 2+ with S 2 o 8 2- The molar ratio is 1:0.6, heated to 65°C in a water bath and stirred for 2 hours to obtain polyferric sulfate.
Embodiment 2
[0018] Taking ferrous sulfate as a soluble ferrous salt as an example, the method for preparing polyferric sulfate is: weigh 13.9g FeSO 4 Placed in a 250mL Erlenmeyer flask, add 50mL of distilled water to FeSO 4 The concentration is 56g / L, add 4.76g Na 2 S 2 o 8 , FeSO 4 with Na 2 S 2 o 8 The molar ratio was 1:0.4, and the reaction was stirred at 25°C for 3 hours to obtain polyferric sulfate.
Embodiment 3
[0020] Taking ferrous sulfate as a soluble ferrous salt as an example, the method for preparing polyferric sulfate is: weigh 13.9g FeSO 4 Placed in a 250mL Erlenmeyer flask, add 50mL of distilled water to FeSO 4 The concentration is 56g / L, add 9.52g Na 2 S 2 o 8 , FeSO 4 with Na 2 S 2 o 8 The molar ratio is 1:0.8, heated to 60°C in a water bath and stirred for 1 hour to obtain polyferric sulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com