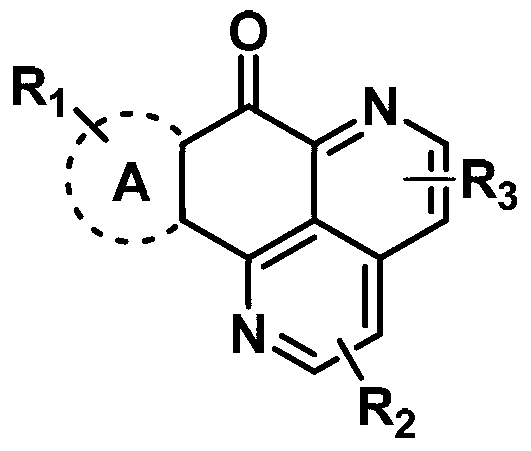
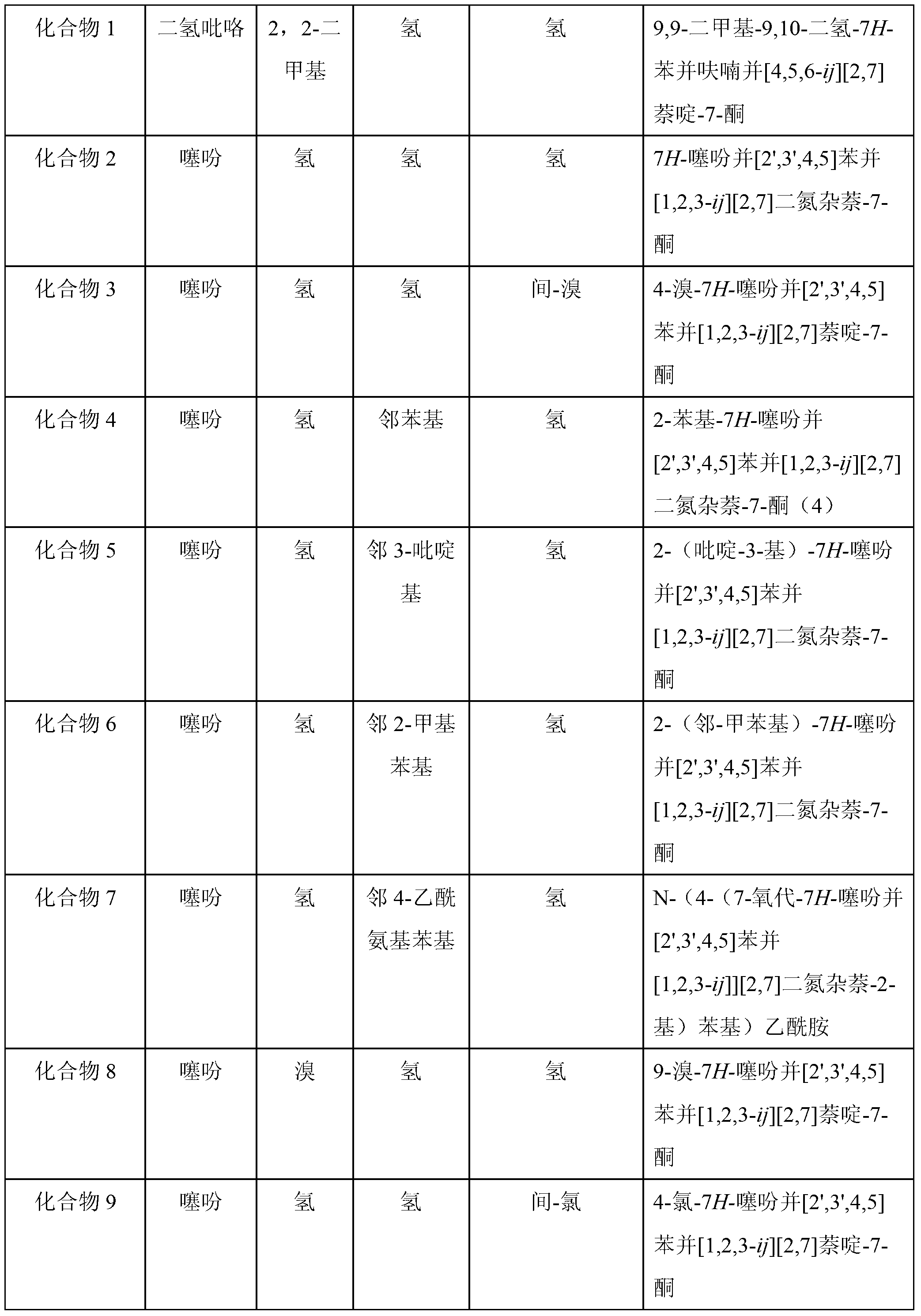
Substituted aryl tetracyclic antifungal compound as well as preparation method and application thereof
A compound, tetracyclic technology, applied in the field of medicine, can solve the problems of antifungal activity and antifungal spectrum to be improved, poor solubility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1: Preparation of (E)-2-((E)-but-2-ene-ylidene)-1,1-dimethylhydrazine (Ⅲ)
[0086] Dissolve (Z)-but-2-enal (7.1g, 0.1mol) in 100mL of anhydrous THF, and slowly add unsymmetrical dimethylhydrazine (6.0g, 0.1mol, 1.0eq) dropwise. After the dropwise addition, the reaction solution was stirred overnight at room temperature. The reaction mixture was concentrated to dryness to obtain 9.8 g of light brown liquid with a yield of 87.5%. 1 H NMR (400MHz, CDCl 3 )δppm:6.96(d,J=8.8Hz,1H),6.12-6.18(dd,J=8.8,12.6Hz,1H),5.73-5.79(m,1H),2.76(s,6H),1.77(d ,J=6.8Hz,3H).
Embodiment 2
[0087] Example 2: Preparation of 4,6-dibromo-2,2-dimethyl-2,3-dihydrobenzofuran-7-ol (Ⅴ)
[0088] Dissolve 1 g of the raw material 2,2-dimethyl-2,3-dihydrobenzofuran-7-ol (IV) (6.0 mmol) in chloroform (35 mL) and cool to 0°C. Liquid bromine (1g, 15mmol, 2.5eq) was slowly added dropwise. After removing the ice bath, react at room temperature for 2 hours. After concentration and removal of the solvent, a brown solid was obtained. After column chromatography (dichloromethane as eluent), 1.4 g of the product was obtained, with a yield of 67.3%. 1 H NMR (400MHz, CDCl 3 )δppm: 6.98(s,1H), 4.99(br s,1H), 3.00(s,2H), 1.44(s,6H).
Embodiment 3
[0089] Example 3: Preparation of 6-bromo-2,2-dimethyl-2,3-dihydrobenzofuran-4,7-dione (Ⅵ)
[0090]Dissolve 470 mg of chromium trioxide (4.7 mmol) in water (5 mL). This solution was added dropwise to a volume ratio of 7 containing 4,6-dibromo-2,2-dimethyl-2,3-dihydrobenzofuran-7-ol (Ⅴ) (500mg, 1.6mmol): 2 in acetic acid-water solution (23mL). After reacting for 1 hour, add 50 mL of water to dilute, and extract 3 times with ethyl acetate. The organic phases were combined, dried and concentrated to obtain a crude product. After silica gel column chromatography (eluent: petroleum ether: ethyl acetate = 1:5), 220 mg of red powdery compound was obtained, with a yield of 52%. 1 HNMR (400MHz, CDCl 3 )δppm: 7.01(s,1H), 2.85(s,2H), 1.49(s,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com