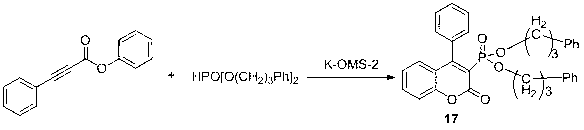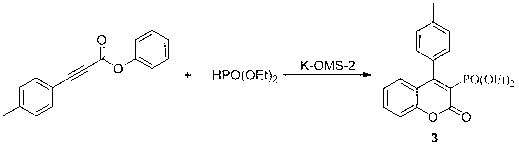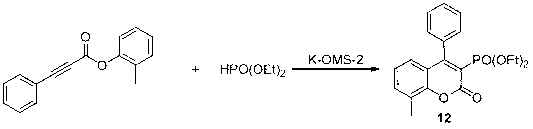Preparation method of 4-aryl coumarin-3-phosphonate derivatives
A technology for aryl coumarin and aryl propynoic acid aryl ester, which is applied in the field of coumarin derivatives, can solve the problems of small scope of application and long reaction time, and achieves short reaction time, high yield, and high reaction time. mild effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of 4-phenyl-2H-benzopyran-2-one-3-phosphonate
[0032] Using 3-phenylpropiolic acid phenyl ester and dimethyl phosphite as raw materials, the reaction formula is as follows:
[0033]
[0034] (1) Add 0.22 g (1 mmol) of phenyl 3-phenylpropiolate, 0.11 g (1 mmol) of dimethyl phosphite, 0.1 g of K-OMS-2 and methanol (30 mL) into the reaction flask , 20°C reaction;
[0035] (2) TLC tracking reaction until completely finished;
[0036] (3) The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 75%).
[0037] 1 H NMR (400 MHz, CDCl 3 ): δ 7.60 (t, J = 7.59 Hz, 1H, ArH), 7.55 ? 7.50 (m, 3H, ArH), 7.38 (d, J = 8.30 Hz, 1H, ArH), 7.36 ? 7.30 (m, 3H, ArH), 7.18 (t, J = 7.16 Hz, 1H, ArH), 7.06 (d, J = 7.95 Hz, 1H, ArH), 3.60 ? 3.59 ( m, 3H, CH 3 ), 3.57 ? 3.55 ( m, 3H, CH 3 );HRMS (EI): m / z (%) calcd. for C 17 h 15 o 5 P, 330.0657, ...
Embodiment 2
[0038]Example 2: Synthesis of 4-phenyl-2H-benzopyran-2-one-3-phosphonate
[0039] With phenyl 3-phenylpropiolate and diethyl phosphite as raw materials, the reaction formula is as follows:
[0040]
[0041] (1) Add 0.22 g (1 mmol) of phenyl 3-phenylpropiolate, 0.21 g (1.5 mmol) of diethyl phosphite, 0.05 g of K-OMS-2 and ethanol (30 mL) into the reaction flask , 40°C reaction;
[0042] (2) TLC tracking reaction until complete completion;
[0043] (3) The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 82%).
[0044] 1 H NMR (300 MHz, CDCl 3 ): δ 7.55 ? 7.45 (m, 3H, ArH), 7.38 (s, 1H, ArH), 7.34 ? 7.28 (m, 2H, ArH), 7.12 (d, J = 6.7 Hz, 2H, ArH), 6.97 (d, J = 8.6 Hz, 1H, ArH), 4.15 ? 4.00 (m, 2H, CH 2 ), 4.0 ? 3.82 (m, 2H, CH 2 , 1.11 (m, 6H, CH 3 ); HRMS (EI, M + ): calcd for C 19 h 19 o 5 P 358.0971, found 358.1043.
Embodiment 3
[0045] Example 3: Synthesis of 4-(4-methylphenyl)-2H-benzopyran-2-one-3-phosphonate
[0046] Using 3-(4-methylphenyl)phenyl propiolate and diethyl phosphite as raw materials, the reaction formula is as follows:
[0047]
[0048] (1) Add 0.24 g (1 mmol) of 3-(4-methylphenyl) phenyl propiolate, 0.28 g (2 mmol) of diethyl phosphite, and 0.1 g of K-OMS-2 into the reaction flask React with acetonitrile (30 mL) at 50°C;
[0049] (2) TLC tracking reaction until complete completion;
[0050] (3) The crude product obtained after the reaction was separated by column chromatography (petroleum ether: ethyl acetate = 4:1) to obtain the target product (yield 87%).
[0051] 1 H NMR (300 MHz, CDCl 3 ): δ 7.52 (s, 1H, ArH), 7.35 ? 7.24 (m, 3H, ArH), 7.20 ? 7.04 (m, 4H, ArH), 4.10-4.00 (m ,2H, CH 2 ), 3.96 ? 3.84 (m, 2H, CH 2 ), 2.40 (s, 3H, CH 3 ), 1. 08 (m, 6H, CH 3 ); HRMS (EI, M + ): calcd for C 20 h 21 o 5 P 372.1127, found 372.1205 。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com