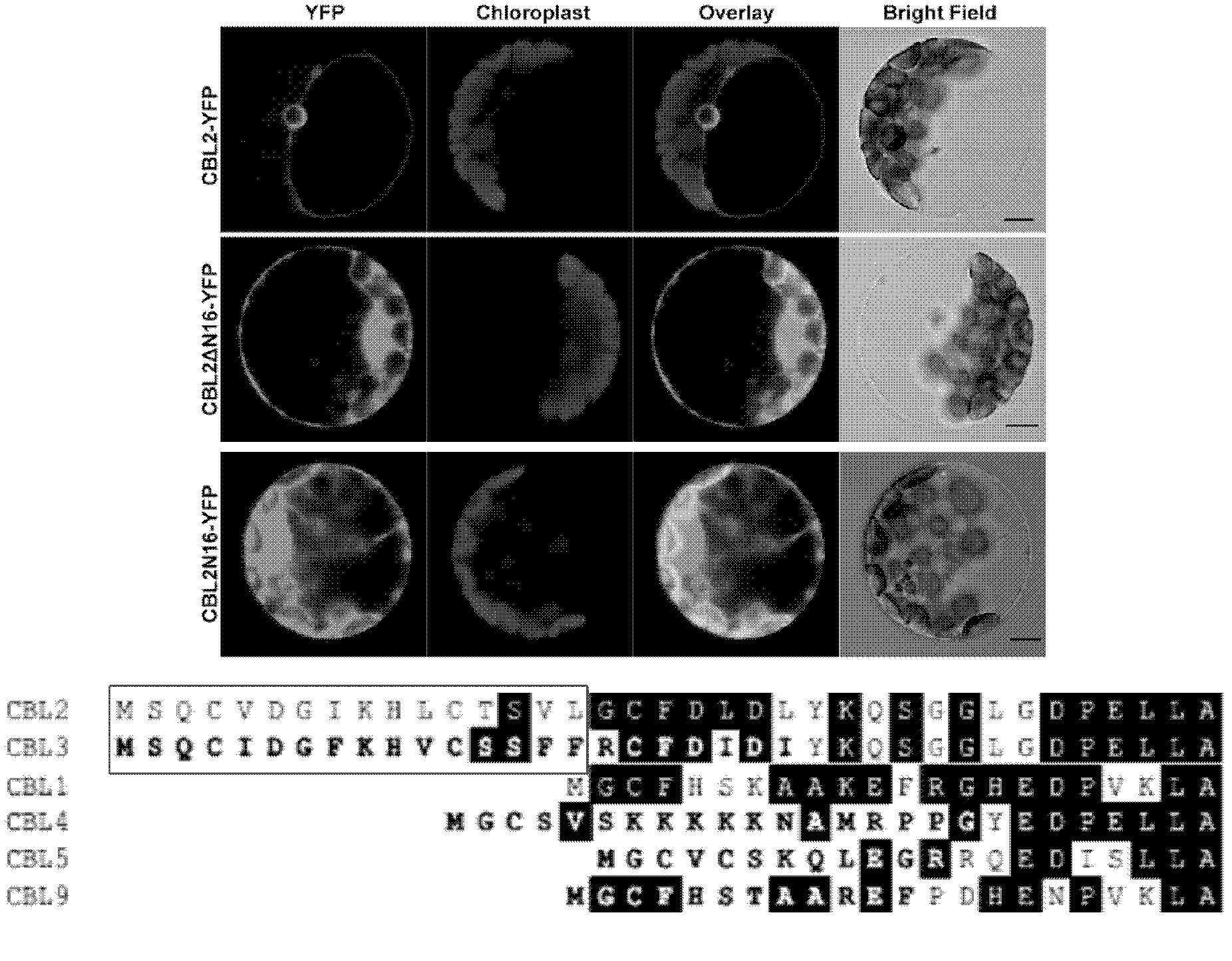Tonoplast localization sequence and its application
A technology of tonoplast membrane and sequence, applied in the fields of biotechnology and botany
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1, the cloning of gene and the construction of recombinant vector
[0095] 1. Cloning of CBL2 and CBL3 genes
[0096] The 14-day-old wild-type Arabidopsis (Col-0) RNA was extracted, reverse-transcribed into cDNA by ReverTra Ace reverse transcriptase (TOYOBO, Japan), and the gene sequences of CBL2 and CBL3 were respectively amplified using the cDNA as a template. The primers used are as follows:
[0097] CBL2F: CTAATG TCGCAGTGCGTTGACGGT (SEQ ID NO: 7);
[0098] CBL2R: GCCGCTGCTTGCTTTTGCTTTTG (SEQ ID NO: 8);
[0099] CBL3F: CATATG TCGCAGTGCATAGACGGT (SEQ ID NO: 9);
[0100] CBL3R:TTCCCAAATTGTCTCCTCTGCTAA (SEQ ID NO: 10).
[0101] The full-length gene sequences were ligated into the EcoRV digested pBluescript KS (pKS) plasmid (Invitrogen) and sequenced.
[0102] 2. Construction of CBL2-YFP and CBL3-YFP vectors and acquisition of transgenic plants
[0103] Using the pKS plasmids containing CBL2 and CBL3 as templates, high-fidelity Taq enzyme KOS-plus wa...
Embodiment 2
[0138] Embodiment 2, cell localization
[0139] The CBL2 and CBL3 genes in Arabidopsis thaliana were isolated and cloned by molecular cloning technology, and the recombinant vectors for transient expression of CBL2-YFP and CBL3-YFP were constructed.
[0140] The method of PEG-mediated transformation of plant leaf protoplasts (Yoo SD, Cho YH, Sheen J (2007); Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565-1572) in Arabidopsis 35S:CBL2-YFP and 35S:CBL3-YFP were transiently expressed in mustard protoplasts. Then the position of the fused fluorescent protein was observed under a fluorescent confocal microscope.
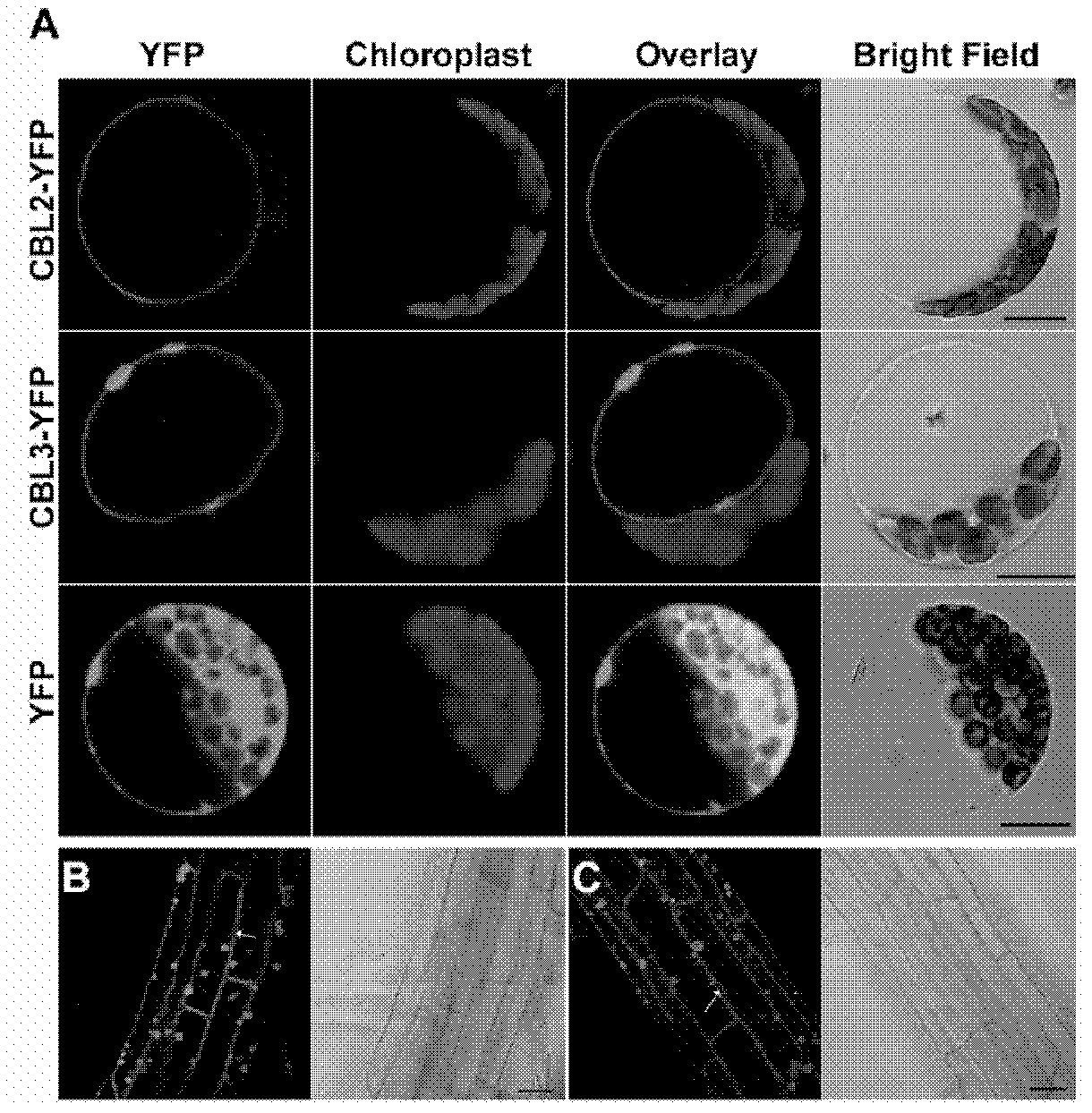
[0141] The results showed that the signals of 35S:CBL2-YFP and 35S:CBL3-YFP were clearly localized on the tonoplast membrane of Arabidopsis cells, as shown in figure 1 a.
[0142] In order to exclude the effects of transient expression and high osmotic potential environment, the inventors constructed...
Embodiment 3
[0143] Example 3, Tonoplast Membrane Localization Related Sequences
[0144] Sequence comparison shows that CBL2 and CBL3 have 16 more amino acids than plasma membrane localized proteins such as CBL1 in the same family, which may be very important for its tonoplast localization ( figure 2 Figure below, box marked). Since CBL2 and CBL3 proteins are highly homologous, the inventors further took CBL2 as an object to study the determinants of their tonoplast membrane localization. A recombinant vector expressing the N-terminal 16 amino acids of CBL2 and YFP fusion protein (CBL2N16-YFP) and a recombinant vector expressing CBL2 and YFP fusion protein (CBL2ΔN16-YFP) without the N-terminal 16 amino acids of CBL2 were constructed. After transient expression in Arabidopsis protoplasts, the position of the fused fluorescent protein was visualized under a fluorescent confocal microscope.
[0145] It was found that after removing the 16 amino acids at the front end of CBL2, the protein ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com