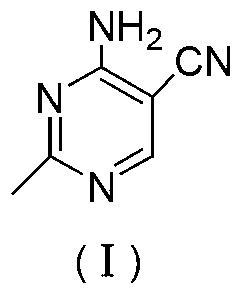
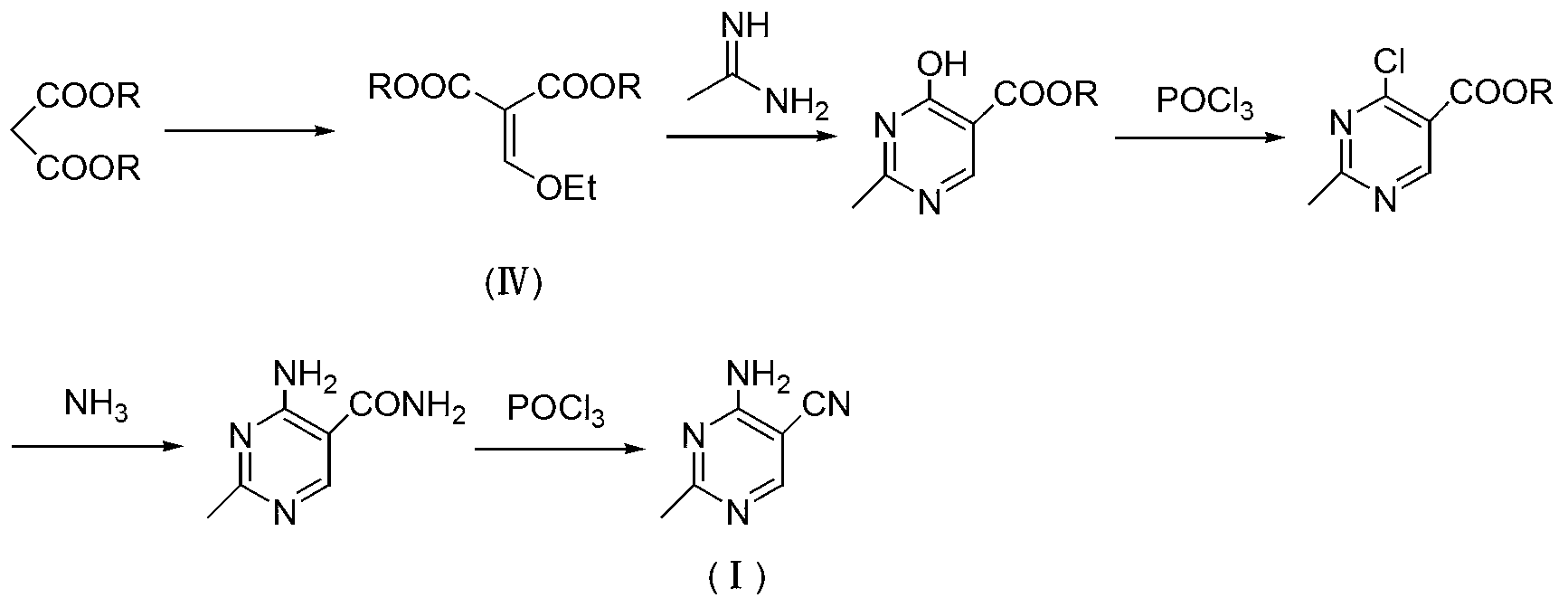
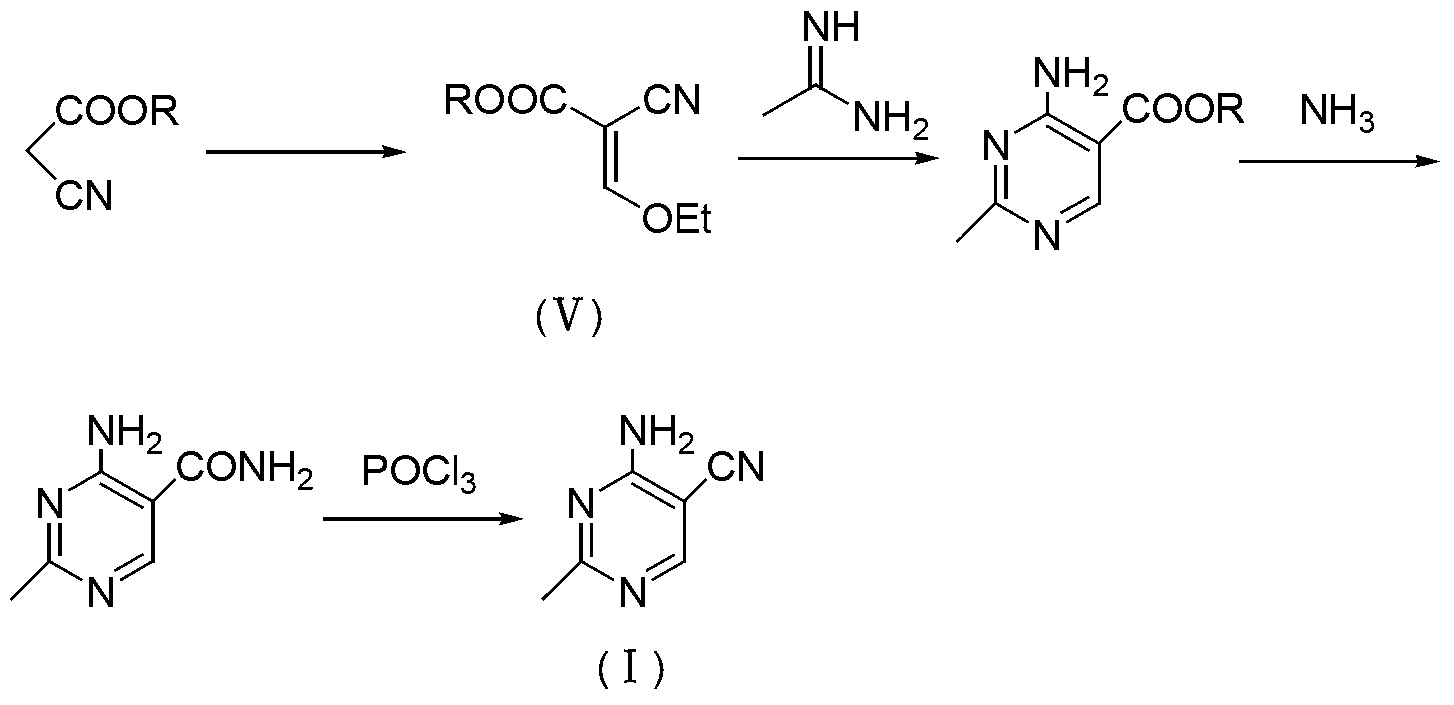
Method for preparation of 2-ethyl-4-amtno-5-cyanopyrimidine
A technology of cyanopyrimidine and amino, which is applied in the field of preparation of 2-methyl-4-amino-5-cyanopyrimidine, and achieves the effects of high yield, short process route and convenient industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Put cyanoacetamide (50g, 0.6mol), N,N-dimethylformamide (91g, 1.25mol) and pyridine (4.7g, 0.06mol) in a dry reaction flask, cool down to -10°C, and Phosphorus oxychloride (193 g, 1.25 mol) was added dropwise within 3 hours, and the mixture was stirred at -10°C for 12 hours after the drop was completed. After the reaction was completed, the mixture was poured into ice water (700mL), adjusted to pH=3, extracted with dichloromethane (300ml×3), the extracts were combined and concentrated to give orange oil (II) (R 1 =R 2 =CH 3 , 54g, 74%), the purity is 98% (GC).
[0029] Cool the methanol solution (400mL) of sodium methoxide (26.5g, 0.49mol) to 0°C, add acetamidine hydrochloride (46.3g, 0.49mol) in batches, stir at 0°C for 20min, filter, and pour the filtrate into the above oil Stir at 25°C for 12h, filter, wash with methanol (3×20mL), and dry in vacuo (50°C, 2h, -0.1MPa) to obtain 51.8g of white solid, yield 65% (calculated as cyanoacetamide), Purity 99% (GC).
[00...
Embodiment 2
[0033] Put cyanoacetamide (50g, 0.6mol), N,N-dimethylacetamide (126.4g, 1.25mol), pyridine (4.7g, 0.06mol) in a dry reaction flask, cool down to -10°C, Phosphorus oxychloride (193 g, 1.25 mol) was added dropwise within 3 h, and the mixture was stirred at -10°C for 12 h after the drop was completed. After the reaction was completed, the mixture was poured into ice water (700mL), adjusted to pH=3, extracted with dichloromethane (300ml×3), the extracts were combined and concentrated to give orange oil (II) (R 1 =R 2 =C 2 h 5 , 64.4g, 72%) with a purity of 98% (GC).
[0034]Cool the methanol solution (400mL) of sodium methoxide (25.4g, 0.47mol) to 0°C, add acetamidine hydrochloride (44.4g, 0.47mol) in batches, after the addition is complete, stir at 0°C for 20min, filter, and pour the filtrate into the above In the oil, stirred at 25°C for 12h, filtered, washed with methanol (3×20mL), and dried in vacuum (50°C, 2h, -0.1MPa) to obtain 50.2g of white solid, yield 63% (calculated...
Embodiment 3
[0036] Put cyanoacetamide (50g, 0.6mol), N,N-dimethylformamide (91g, 1.25mol) and pyridine (4.7g, 0.06mol) in a dry reaction flask, cool down to -5°C, and Phosphorus oxychloride (193 g, 1.25 mol) was added dropwise within 3 hours, and the mixture was stirred at -5°C for 12 hours after the drop was completed. After the reaction was completed, the mixture was poured into ice water (700mL), adjusted to pH=3, extracted with dichloromethane (300ml×3), the extracts were combined and concentrated to give orange oil (II) (R 1 =R 2 =CH 3 , 49g, 68%), the purity is 98% (GC).
[0037] Cool the methanol solution (400mL) of sodium methoxide (24.4g, 0.45mol) to 0°C, add acetamidine hydrochloride (42.5g, 0.45mol) in batches, stir at 0°C for 20min, filter, pour the filtrate into the above oil Stir at 25°C for 12h, filter, wash with methanol (3×20mL), and dry in vacuo (50°C, 2h, -0.1MPa) to obtain 48.2g of white solid, yield 60% (calculated as cyanoacetamide), Purity 99% (GC). 1 H-NMR and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com