Acceptor organogel for colorimetrically identifying fluorine ions in single-selectivity manner as well as preparation and application of acceptor organogel
An organic gel and fluoride ion technology, applied in the field of anion detection, can solve problems such as difficult synthesis, complex structure, and inability to realize single selective recognition of specific ions, and achieve low cost and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
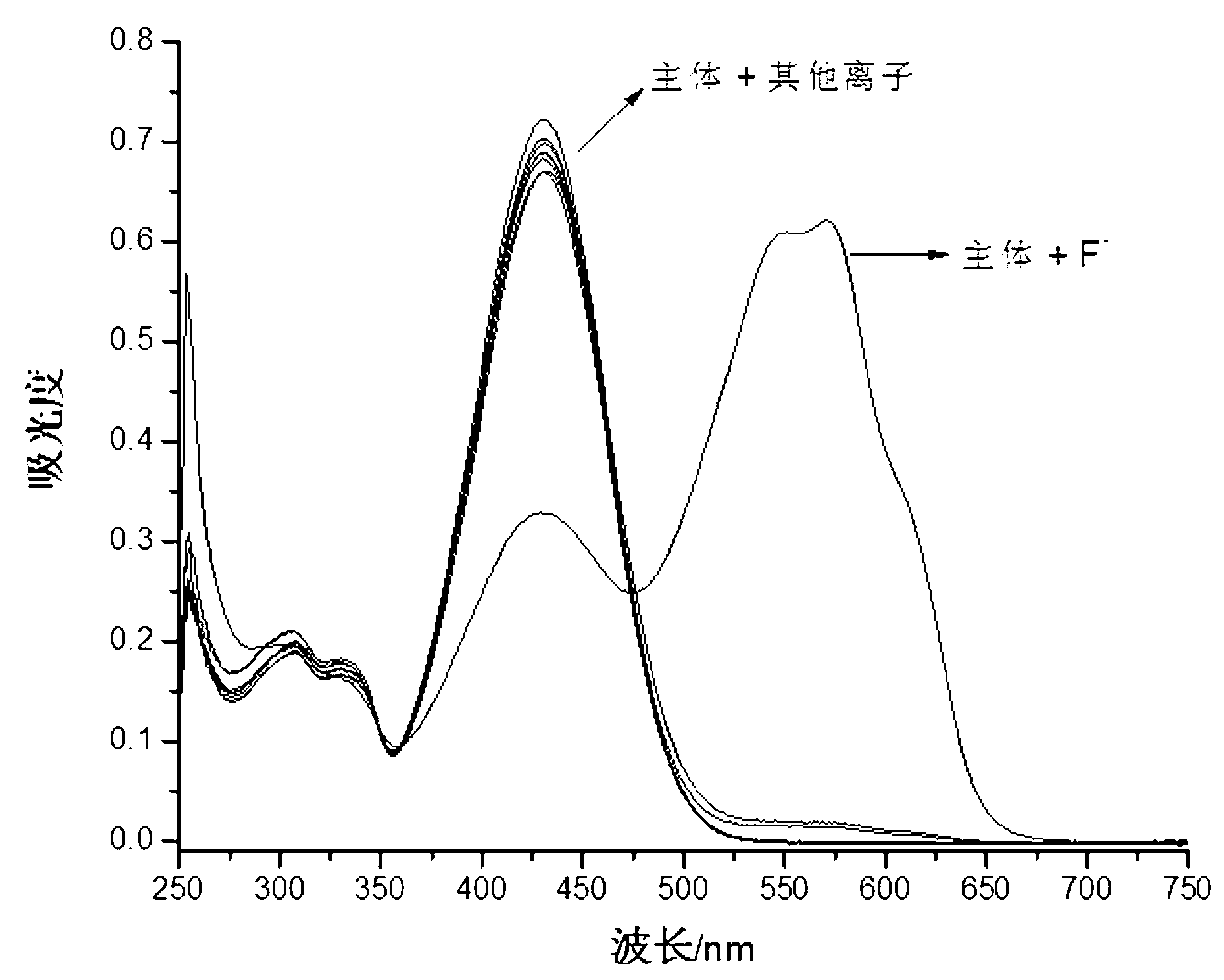
Image
Examples
Embodiment Construction
[0031] 1. Preparation of fluoride ion acceptor
[0032] (1) Preparation of intermediate 1-3,4-bis(hexadecyloxy)benzaldehyde
[0033] 5.0 mmol 3,4-dihydroxybenzaldehyde, 20.0 mmol anhydrous K 2 CO 3 , 1.0 mmol KI and 30 mL acetone were mixed and heated to reflux for 2h, then 1-bromo-n-hexadecane 10.0 mmol was slowly added dropwise, and refluxed for 48h. After the reaction was complete, evaporate the acetone to dryness, add CHCl 3 Extract 3 times, filter, wash the filtrate once with distilled water, wash 2 times with 2% NaOH solution, wash 3 times with saturated NaCl solution, and finally wash with anhydrous NaOH solution 2 SO 4 dry. Rotary evaporation to remove CHCl 3 The product 1-3,4-bis(hexadecyloxy)benzaldehyde (this method refers to Tatsuya Kitahara, Michihiro Shirakawa, Shin-ichiro Kawano, Uwe Beginn, Norifumi Fujita, Seiji Shinkai; J. A. C. S. 2005, 127, 14980. ).
[0034] (2) Preparation of fluoride ion acceptor
[0035] Add 5.0 mmol of 1-3,4-bis(hexadecyloxy)b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com