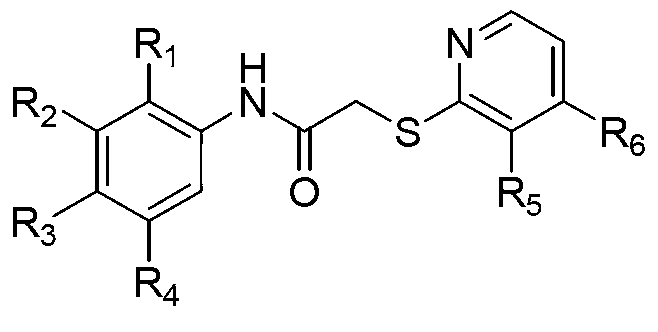
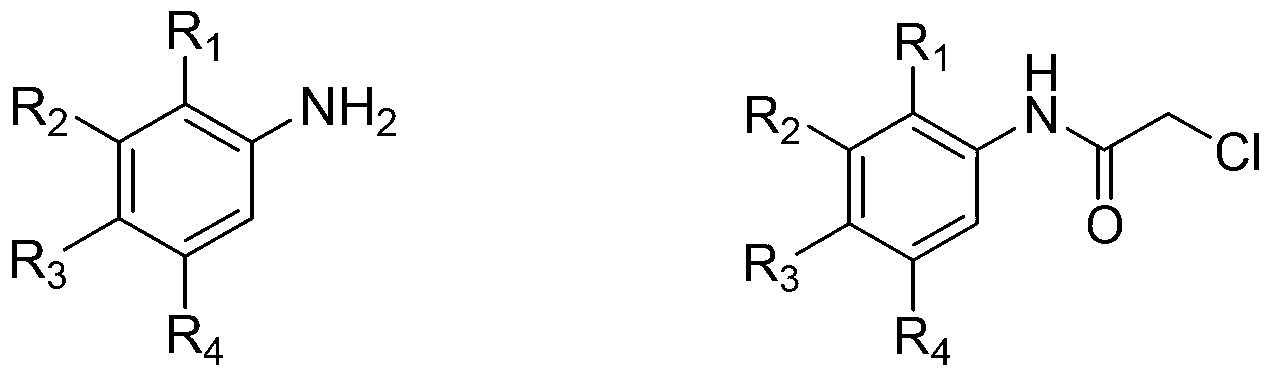
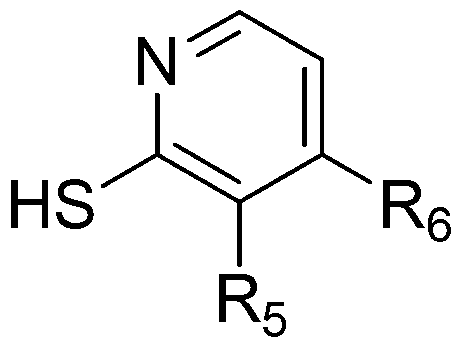
Mercaptonicotinic acid compounds and preparation method thereof
A technology of mercaptonicotinic acid and compound, applied in the field of medicine, can solve problems such as activity improvement, cross-inhibition effect to be improved, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1, the synthesis of 2-(2-(5-chloro-2-methoxyanilino) acetylthio) sodium nicotinate (formula Ⅰ-1)
[0076]
[0077] (Formula Ⅰ-1)
[0078] Dissolve 3.14 g (0.02 mol) of 2-methoxy-5-chloroaniline in 30 ml of glacial acetic acid, add dropwise 2.5 g (0.02 mol) of chloroacetyl chloride, and stir overnight at room temperature. Then the reaction solution was poured into 50 ml of ice water, stirred for 1 hour, a solid was precipitated, filtered, washed with water, and dried to obtain a crude product.
[0079] Add the product from the previous step and 1.55 grams (0.01mol) of 2-mercaptonicotinic acid into 25 milliliters of THF and water mixed solution (volume ratio 1:1), cool to 0 degrees, add 1.6 grams of sodium hydroxide, stir the reaction, and raise the temperature After stirring overnight at room temperature, a large amount of solids precipitated, filtered, washed with water, and dried to obtain 3.55 g of off-white solids with a yield of 86%.
[0080] The res...
Embodiment 2
[0083] Embodiment 2, the synthesis of 2-(2-(5-chloro-2-methoxyanilino) acetylthio) nicotinic acid (formula Ⅰ-2)
[0084]
[0085] (Formula Ⅰ-2)
[0086] Put sodium 2-(2-(5-chloro-2-methoxyanilino)-2-acetylthio)nicotinate prepared in Example 1 in water, then adjust the pH value to 2 with 1M hydrochloric acid, and filter , washed with water, and dried to obtain a white solid with a yield of 97%.
[0087] The structure confirmation results are as follows: 1H-NMR (DMSO-d6): 13.56 (s, 1H), 9.75 (s, 1H), 8.69-8.70 (m, 1H), 8.29-8.30 (m, 1H), 8.19 (d, 1H),7.35-7.37(m,1H),7.01-7.08(m,2H),4.02(s,2H),3.79(s,3H).EI-MS:m / z:375.4[M+H]+ .
[0088] After identification, the obtained product is indeed the target compound formula Ⅰ-2.
Embodiment 3
[0089]Example 3, Synthesis of 2-(2-(5-chloro-2-methoxyanilino) acetylthio) potassium nicotinate (formula Ⅰ-3)
[0090]
[0091] (Formula Ⅰ-3)
[0092] Dissolve 3.14 g of 2-methoxy-5-chloroaniline in 30 ml of glacial acetic acid, add dropwise 2.5 g of chloroacetyl chloride, stir overnight at room temperature, then pour the reaction solution into 50 ml of ice water, stir for 1 hour, and precipitate a solid , filtered, washed with water, and dried to obtain a crude product.
[0093] Add the product from the previous step and 1.55 grams of 2-mercaptonicotinic acid into 25 milliliters of THF and water mixed solution (volume ratio 1:1), cool to 0 degrees, add 2.0 grams of potassium hydroxide, stir the reaction, warm up to room temperature, and stir Overnight, a large amount of solids precipitated, filtered, washed with water, and dried in vacuo to obtain 2.62 g of off-white solids with a yield of 67%.
[0094] The results of structural confirmation are as follows:
[0095] 1H-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com