Fasciolopsis PCR-DHPLC (polymerase chain reaction-denaturing high performance liquid chromatography) detection primers, kit and detection method
A PCR-DHPLC, detection kit technology, applied in biochemical equipment and methods, recombinant DNA technology, microbial determination/inspection, etc., can solve the problems of cumbersome detection steps, high detection cost, easy to cause pollution, etc. Detection efficiency and sensitivity, wide application prospects, labor saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] 1. Template Preparation
[0046] The template DNA extraction methods described below are routine operations of those skilled in the art, and all reagents and solutions are prepared by conventional means or obtained from commercial means.
[0047] ① Add 400 μL DNA extraction buffer and 40 μL proteinase K (10 mg / mL) to the 1.5 mL centrifuge tube containing the sample, mix well and digest at 37 °C for 3 h;
[0048] ②Centrifuge at 10000g for 2min, discard the supernatant; add an equal volume of phenol, chloroform, isoamyl alcohol (25:24:1), mix the organic phase and aqueous phase by vigorous shaking, centrifuge at 10000g, 4°C for 10min, transfer the supernatant to another in a centrifuge tube.
[0049] ③ Take the supernatant of equal volume, add chloroform and isoamyl alcohol (24:1) to shake, centrifuge at 12000g for 10min; extract once.
[0050] ④ Take the supernatant and add pre-cooled 2 times the volume of ethanol (or add an equal volume of isopropanol), shake the mixt...
Embodiment 2
[0079] Embodiment 2 specificity test
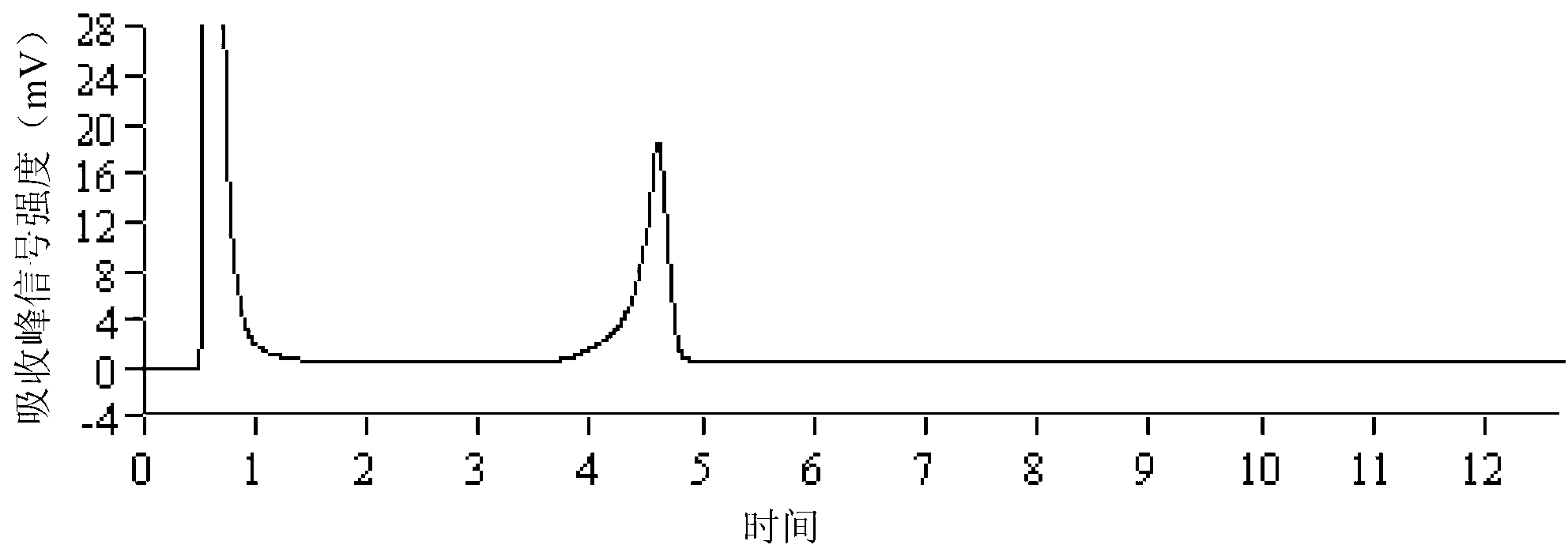
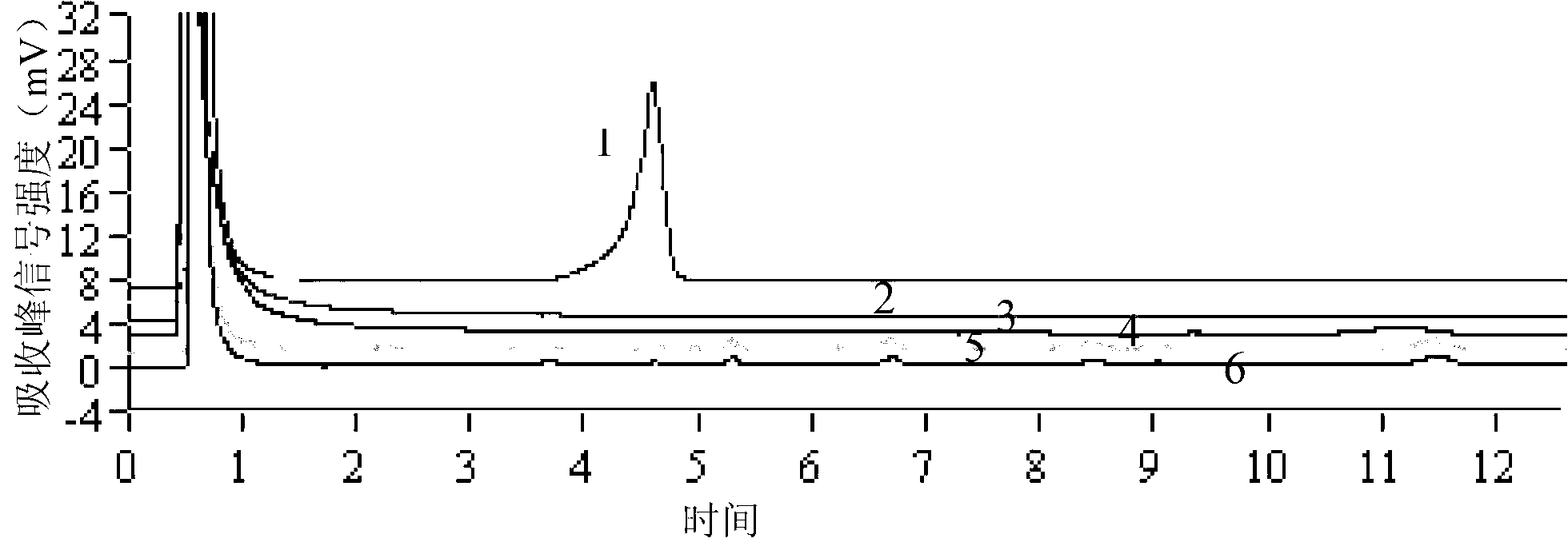
[0080] Samples of parasites such as Fasciola metacercariae, cysticerci, roundworm eggs, Trichinella spiralis, Toxoplasma gondii, and liver fluke were taken, and genomic DNA was extracted according to the method described in Example 1. Using these genomic DNAs as templates, PCR amplification and DHPLC detection were performed according to the method described in Example 1. The results are attached figure 2 As shown, 1-6 are in order: Fasciola metacercariae, cysticerci, roundworm eggs, trichinella spiralis, toxoplasma gondii, liver fluke; only the samples of Fasciola metacercariae have typical PCR product absorption peaks, and the absorption peak is greater than 3mV. The result of this sample was judged to be positive, and Fasciola fragrans was detected. Other parasite samples such as cysticercus, roundworm eggs, trichinella spiralis, toxoplasma gondii, and liver fluke were all negative, and there were no false positive and false negativ...
Embodiment 3
[0081] Embodiment 3 detection sensitivity test
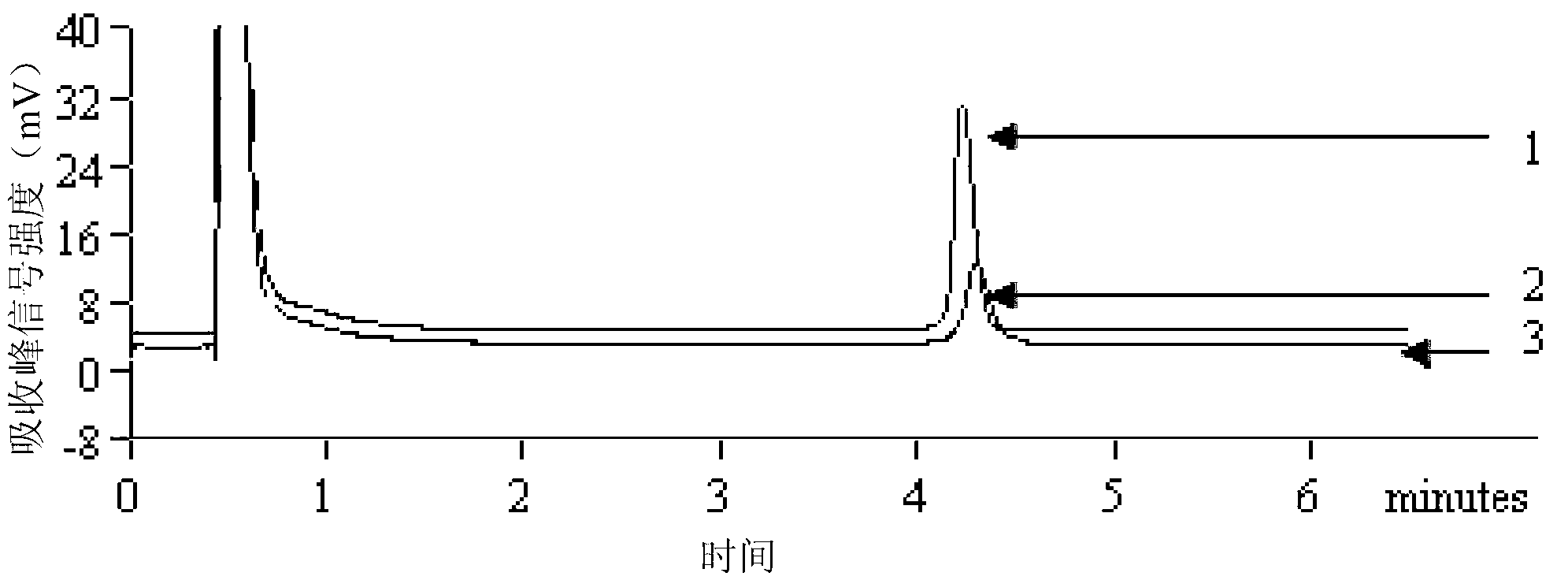
[0082] A sample of the metacercariae of Fasciola zingiberi was taken, and the count under the microscope was 1.06×10 2 cells / mL, and then diluted successively to 1.06×10 cells / mL, 1.06 cells / mL, 1.06×10 cells / mL -1 individual / mL. 10 μL of each dilution was added to a PCR reaction tube, and DNA was extracted according to the method established in Example 1. Take 2 μL each as a template, and perform PCR-DHPLC detection according to the method established in Example 1. The results are attached image 3 Shown: 1. 1.06×10 metacercariae / mL; 2. 1.06 metacercariae / mL; 3. 1.06×10 metacercariae -1 individual / mL. The results showed that the sensitivity of this method was very high, and about 1 / mL (g) metacercariae of Fasciola zingiberi could be detected.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com