Method for co-production of trifluoroethylene and hydrogen fluoride
A technology of trifluoroethylene and hydrogen fluoride, which is applied in the fields of fluorine/hydrogen fluoride, chemical instruments and methods, and preparation of dehydrohalogenation, etc., and can solve problems such as potential safety hazards, insufficient utilization of anhydrous hydrogen fluoride, and no economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
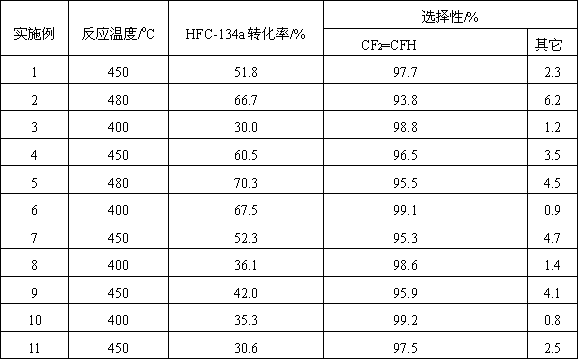
Examples
Embodiment 1
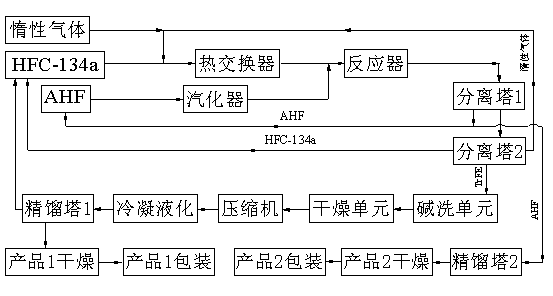
[0033] refer to figure 1 : A certain amount of α-Al 2 o 3 into the reactor bed. According to HFC-134a and CO 2 The molar ratio is 1:5, HFC-134a and CO are fed 2 The gas forms a mixed gas, and enters the reactor after passing through the heat exchanger. -1 The reaction is carried out at the space velocity; the reacted gas is recycled through the HF gas separated by the first separation tower, and the HF gas can also be separated through the second rectification tower, and then dried to obtain HF gas with a purity of more than 99.9%. The separated tail gas is further separated through the separation tower, TrFE, HFC-134a, CO 2 Gas and other trace components enter the second separation tower for further separation, HFC-134a and CO 2Gas recycling, the separated TrFE and other trace components are washed and dried with alkali, then compressed by a compressor, condensed and liquefied, and then enter a rectification tower for rectification. The trace non-condensable gas can be ...
Embodiment 2
[0036] refer to figure 1 : A certain amount of α-Al 2 o 3 Put it into the reactor bed, vaporize AHF in the vaporization chamber, and fluoride at 260°C and 400°C for 2 and 4 hours, respectively. According to HFC-134a and N 2 The molar ratio is 1:5, HFC-134a and CO are fed 2 The gas forms a mixed gas, and enters the reactor after passing through the heat exchanger. -1 The reaction is carried out at the space velocity; the reacted gas is recycled through the HF gas separated by the first separation tower, and the HF gas can also be separated through the second rectification tower, and then dried to obtain HF gas with a purity of more than 99.9%. The separated tail gas is further separated through the separation tower, TrFE, HFC-134a, CO 2 Gas and other trace components enter the second separation tower for further separation, HFC-134a and CO 2 Gas recycling, the separated TrFE and other trace components are washed and dried with alkali, then compressed by a compressor, cond...
Embodiment 3
[0039] refer to figure 1 : A certain amount of γ-Al 2 o 3 into the reactor bed. According to HFC-134a and CO 2 The molar ratio is 1:9, HFC-134a and CO are fed 2 The gas forms a mixed gas, and then enters the reactor after passing through the heat exchanger. -1 The reaction is carried out at the space velocity; the reacted gas is recycled through the HF gas separated by the first separation tower, and the HF gas can also be separated through the second rectification tower, and then dried to obtain HF gas with a purity of more than 99.9%. The separated tail gas is further separated through the separation tower, TrFE, HFC-134a, CO 2 Gas and other trace components enter the second separation tower for further separation, HFC-134a and CO 2 Gas recycling, the separated TrFE and other trace components are washed and dried with alkali, then compressed by a compressor, condensed and liquefied, and then enter a rectification tower for rectification. The trace non-condensable gas c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com