Active material for nonaqueous electrolyte secondary battery, method for production of the active material, electrode for nonaqueous electrolyte secondary battery and nonaqueous electrolyte secondary battery
A non-aqueous electrolyte and secondary battery technology, which is applied in the direction of non-aqueous electrolyte storage batteries, battery electrodes, secondary batteries, etc., can solve problems such as lithium composite oxide particles that are not specifically recorded, and achieve high-rate discharge characteristics and excellent output characteristics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
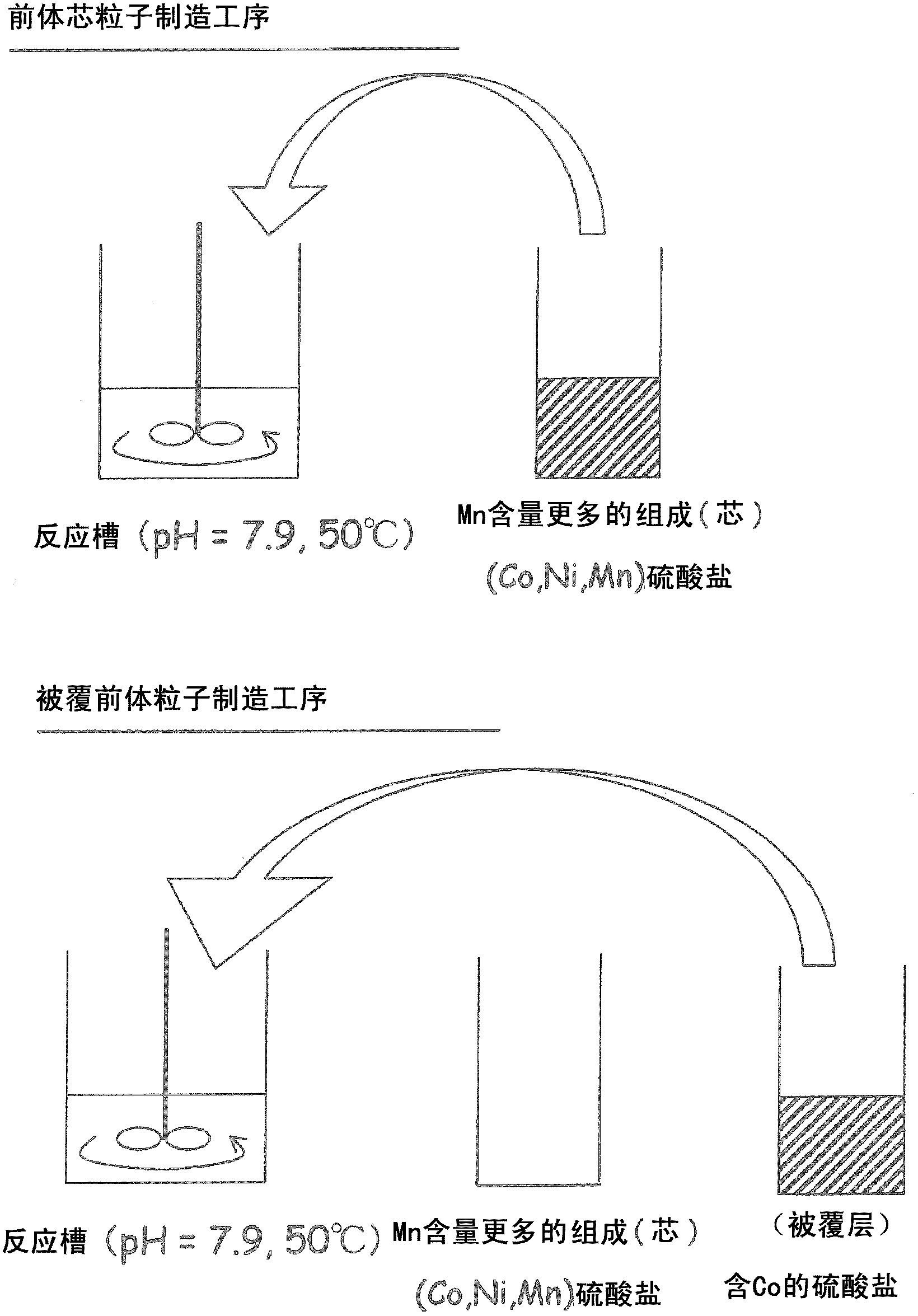
[0050] In Example 1, in order to make the Co concentration of the coating part higher than the Co concentration of the core, it is preferable to use a precursor particle of a transition metal compound coated with a compound containing Co or a precursor of a transition metal compound coated with a compound containing Co and Ni. Particles, or precursor particles of a transition metal compound coated with a compound containing Co, Ni, and Mn and containing more Co than Mn in terms of molar ratio.
[0051]In addition, in order to improve the cycle characteristics of high-rate discharge, it is preferable to use lithium transition metal composite oxide particles in which the Co concentration in one particle continuously changes. Therefore, in Example 2, when the surface position of the particle is represented as 0 and the center position is represented as 1, the starting point of the cobalt concentration gradient region from the particle surface exists at 0.1-0.5. The core is a regi...
Embodiment 2
[0059] In Example 2, in order to improve the output characteristics while improving the high-rate discharge characteristics, it is preferable that the average particle diameter of the lithium transition metal composite oxide particles be 8 μm or less. As shown in Examples described below, in the precursor production process, by setting the stirring duration after the dropwise addition of the raw material aqueous solution to less than 5 hours, the average particle size can be set to 8 μm or less. When the average particle diameter exceeds 8 μm, the initial high-rate discharge capacity of a nonaqueous electrolyte secondary battery using the same becomes small, but the cycle characteristics of high-rate discharge are excellent.
[0060] Next, a method for producing the active material for a nonaqueous electrolyte secondary battery of the present invention will be described.
[0061] The manufacturing method of the active material for non-aqueous electrolyte secondary battery of e...
Embodiment 1-1
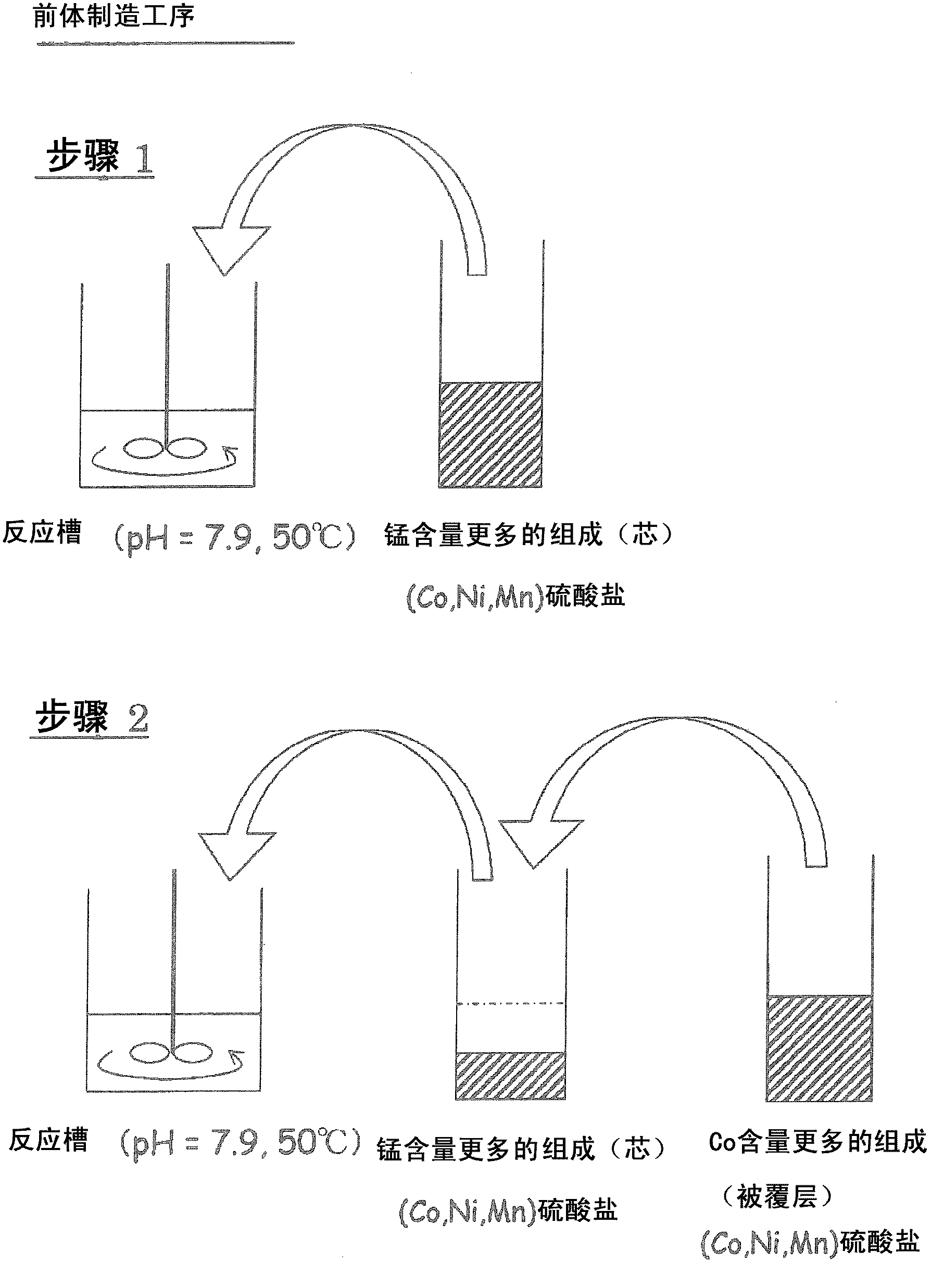
[0111] [Precursor core particle manufacturing process]
[0112] Cobalt sulfate 7 hydrate, nickel sulfate 6 hydrate, and manganese sulfate 5 hydrate were dissolved in 200 ml of ion-exchanged water to produce a 2.00 mol / l sulfate solution with a molar ratio of Co: Ni: Mn of 12.5: 19.94: 67.56 .
[0113] By injecting 750ml of ion exchanged water into a 2L reaction tank, the CO 2 Gas was bubbled for 30 min, so that the CO 2 Dissolved in ion-exchanged water. The temperature of the reaction tank was set at 50° C. (±2° C.), and the reaction tank was stirred at a rotation speed of 700 rpm using a paddle blade equipped with a stirring motor, and the sulfate solution was added dropwise at a rate of 3 ml / min. Among them, from the beginning to the end of the dropping, the aqueous solution containing 2.00 mol / l sodium carbonate solution and 0.4 mol / l ammonia solution is appropriately dropped, so as to control the pH in the reaction tank to maintain 7.9 (± 0.05) for a long time . After...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com