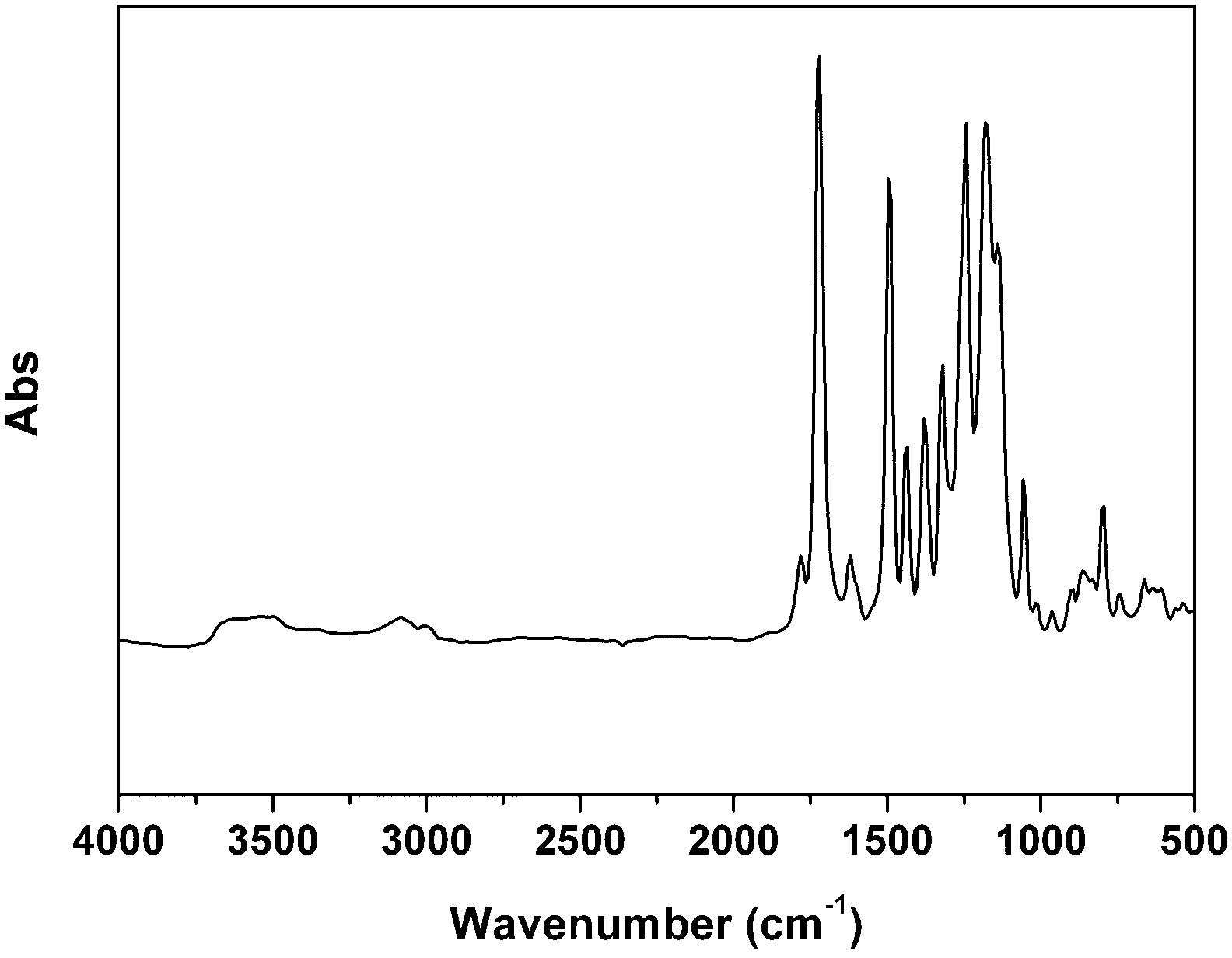
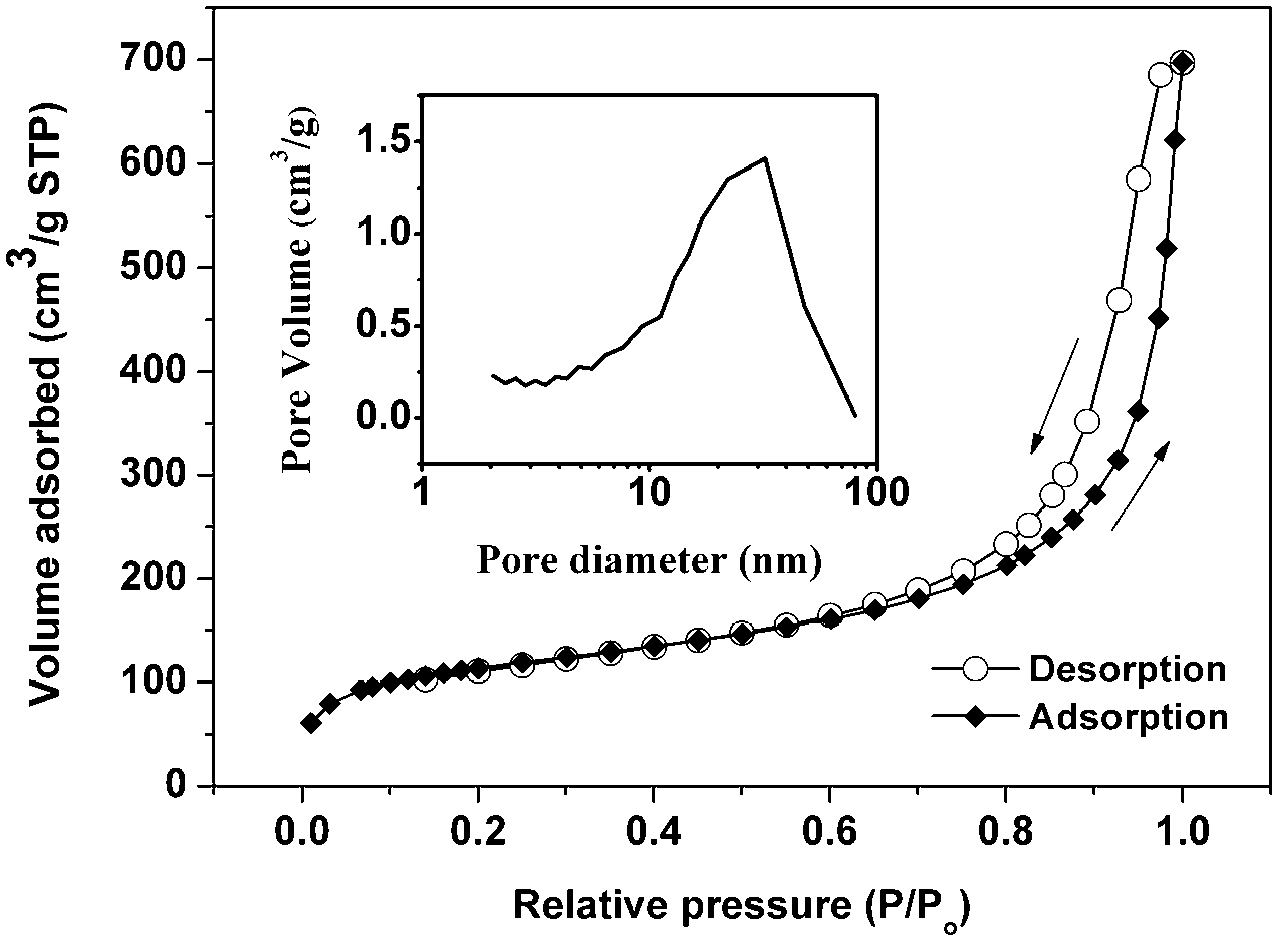
Intrinsic hydrophobic polyimide aerogel and preparation method as well as application thereof
A technology of airgel and polyamic acid, applied in the field of intrinsically hydrophobic polyimide airgel and its preparation, can solve the problem of not effectively improving the hydrophilicity of PI airgel, affecting the application effect and density Problems such as increased dielectric constant and high porosity, low density, low dielectric constant and dielectric loss are achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1) Add 1.9741g (6.16mmol) 2,2'-bis(trifluoromethyl)-4,4'-diaminobiphenyl (TFDB) and 25 g of freshly distilled N-methylpyrrolidone (NMP) was bubbled with nitrogen. After TFDB was completely dissolved, 1.2479g (6.36mmol) of 1,2,3,4-cyclobutanetetracarboxylic dianhydride (CBDA) and 25g of NMP were added, and condensation polymerization was carried out at 25°C for 24h to obtain anhydride-terminated polycarbonate. Amic acid solution.
[0057] 2) Add 0.0574g (0.0497mmol) of amino group-containing polyfunctional capping agent octa(aminophenyl) polysilsesquioxane (OAPS) to the system, and carry out amidation reaction at 25°C for 24h to obtain crosslinked Polyamic acid solution.
[0058] 3) Add 2.52g (31.82mmol) of pyridine and 3.25g (31.82mmol) of acetic anhydride to the cross-linked polyamic acid solution obtained in step 2), stir for 20 minutes, and pour it into a mold for dehydration reaction. The system gels within 4 hours. A gel containing the compound of formula II is ...
Embodiment 2
[0071] 1) Add 1.1102g (6.16mmol) 3,5-diaminotrifluorotoluene (TFMDA) and 25g freshly distilled N-methylpyrrolidone (NMP) in a there-necked flask equipped with mechanical stirring, temperature and nitrogen inlet, Nitrogen was introduced. After TFMDA is completely dissolved, add 1.2479g (6.36mmol) 1,2,3,4-cyclobutanetetraacid dianhydride (CBDA) and 25g of NMP, and carry out condensation polymerization at room temperature (25°C) for 24h to obtain anhydride group Capped polyamic acid solution.
[0072] 2) Add 0.0574g (0.0497mmol) of octa(aminophenyl)polysilsesquioxane (OAPS) to the system in step 1), and carry out amidation reaction at room temperature (25°C) for 24h to obtain a cross-linked polyamide acid solution.
[0073] 3) Add 2.52g (31.82mmol) of pyridine and 3.25g (31.82mmol) of acetic anhydride to the crosslinked polyamic acid solution obtained in step 2). Stir for 20 minutes, pour into a mold for dehydration reaction, and the system gels within 1 hour to obtain a gel c...
Embodiment 3
[0081] 1) Add 1.0858g (6.16mmol) 2,3,5,6-tetrafluoro-p-phenylenediamine (TFPDA) and 25g of freshly distilled N-methyl to a three-neck flask equipped with mechanical stirring, temperature and nitrogen inlet Pyrrolidone (NMP) with nitrogen gas. After TFPDA was completely dissolved, 1.2479g (6.36mmol) of 1,2,3,4-cyclobutane tetra-acid dianhydride (CBDA) and 25g of NMP were added. The polycondensation reaction was carried out at room temperature (25° C.) for 24 hours to obtain an anhydride group-terminated polyamic acid solution.
[0082] 2) Add 0.0574g (0.0497mmol) of octa(aminophenyl)polysilsesquioxane (OAPS) to the system in step 1), and carry out amidation reaction at room temperature (25°C) for 24h to obtain a cross-linked polyamide acid solution.
[0083] 3) Add 2.52g (31.82mmol) of pyridine and 3.25g (31.82mmol) of acetic anhydride to the crosslinked polyamic acid solution obtained in step 2). Stir for 20 minutes, pour into a mold for dehydration reaction, and the system...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com