Rotavirus vaccine for oral administration
A rotavirus and vaccine technology, applied in the direction of antiviral agents, viral antigen components, medical preparations of non-active components, etc., can solve the problems affecting the effectiveness and safety of vaccines, biological hazards, vaccine storage conditions and validity Limitation and other issues to achieve the effect of enhancing gastric acid resistance, good safety, and good immune response stimulation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 rotavirus antigen
[0037]The virus strain UK x D strain (G1), UK x DS-1 provided by the National Institute of Health (hereinafter referred to as NIH) to Beijing Kexing Zhongwei Biotechnology Co., Ltd. as the starting material strain of the vaccine strain (G2), UK x P strain (G3), UK x ST-3 strain (G4), UK x AU32 strain (G9) and UK x Wa strain (P1A[8]). MA-104 cells or diploid cells were adapted to grow and continuously passaged, cultured in a 37.5±1.0°C incubator for 2 to 5 days to harvest the virus liquid, clarified and filtered with CCID 50 The virus titer value was detected by fluorescent quantitative PCR method. The maximum yield of the above six serotypes of rotavirus can reach 10 6 -10 8 CCID 50 / ml. The purified rotavirus stocks of each serotype can be used to prepare vaccine preparations.
Embodiment 2
[0038] The preparation of embodiment 2 rotavirus vaccines
[0039] (1) Prepare 3M phosphate buffer solution (in which the molar concentration ratio of potassium dihydrogen phosphate and dipotassium hydrogen phosphate is 1:2) and 4M sodium succinate solution stock solution, filter and sterilize, and store at 2-8°C for later use;
[0040] (2) Mix 10ml of phosphate buffer prepared in step (1) with 15ml of sodium succinate solution;
[0041] (3) Add 150ml of 60% sucrose solution to (2);
[0042] (4) Add 100ml of the antigen solution in Example 1 to (3), which is a mixture of G1, G2, G3, G4, G9, and P1A[8];
[0043] (5) Supplement the volume to 300ml with virus-free, serum-free MEM culture medium; for vaccines, the pH value is 5-8;
[0044] (6) Filter and dispense, 3ml per dose, 100 doses in total.
[0045] Each dose of the vaccine prepared in this embodiment contains the following ingredients: 10 each of the above-mentioned 6 serotypes of rotaviruses 5 -10 7 CCID 50 , 30% su...
Embodiment 3
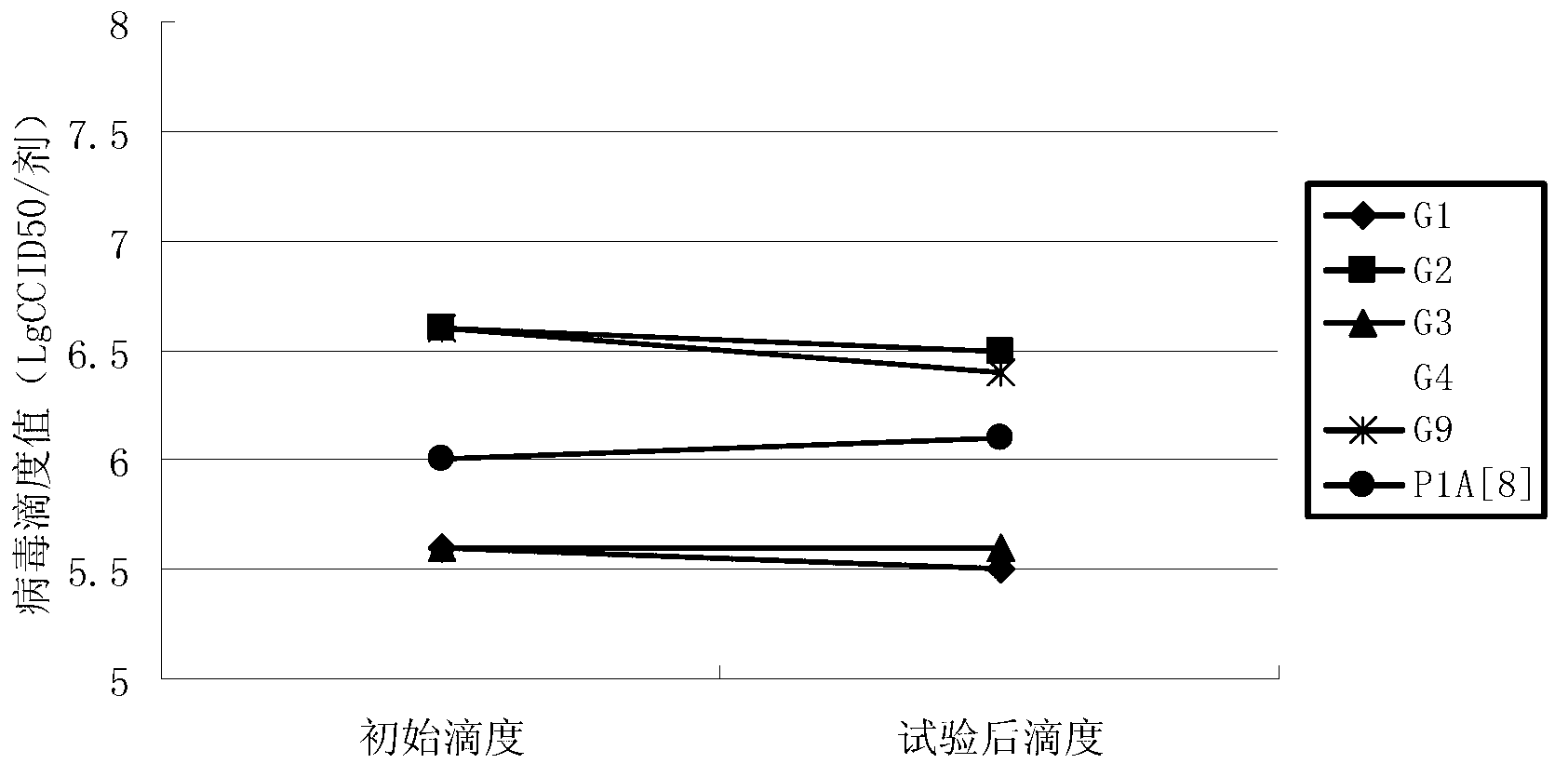
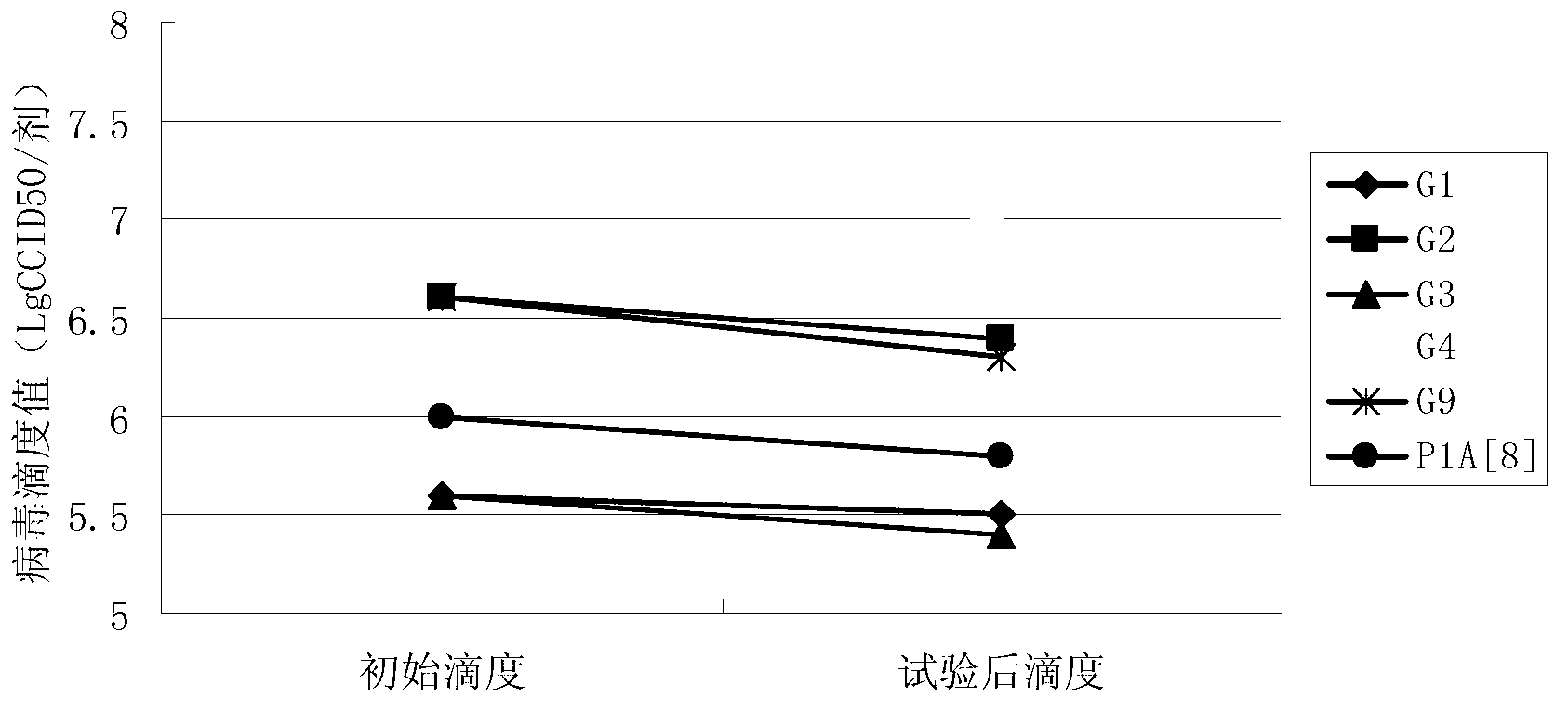
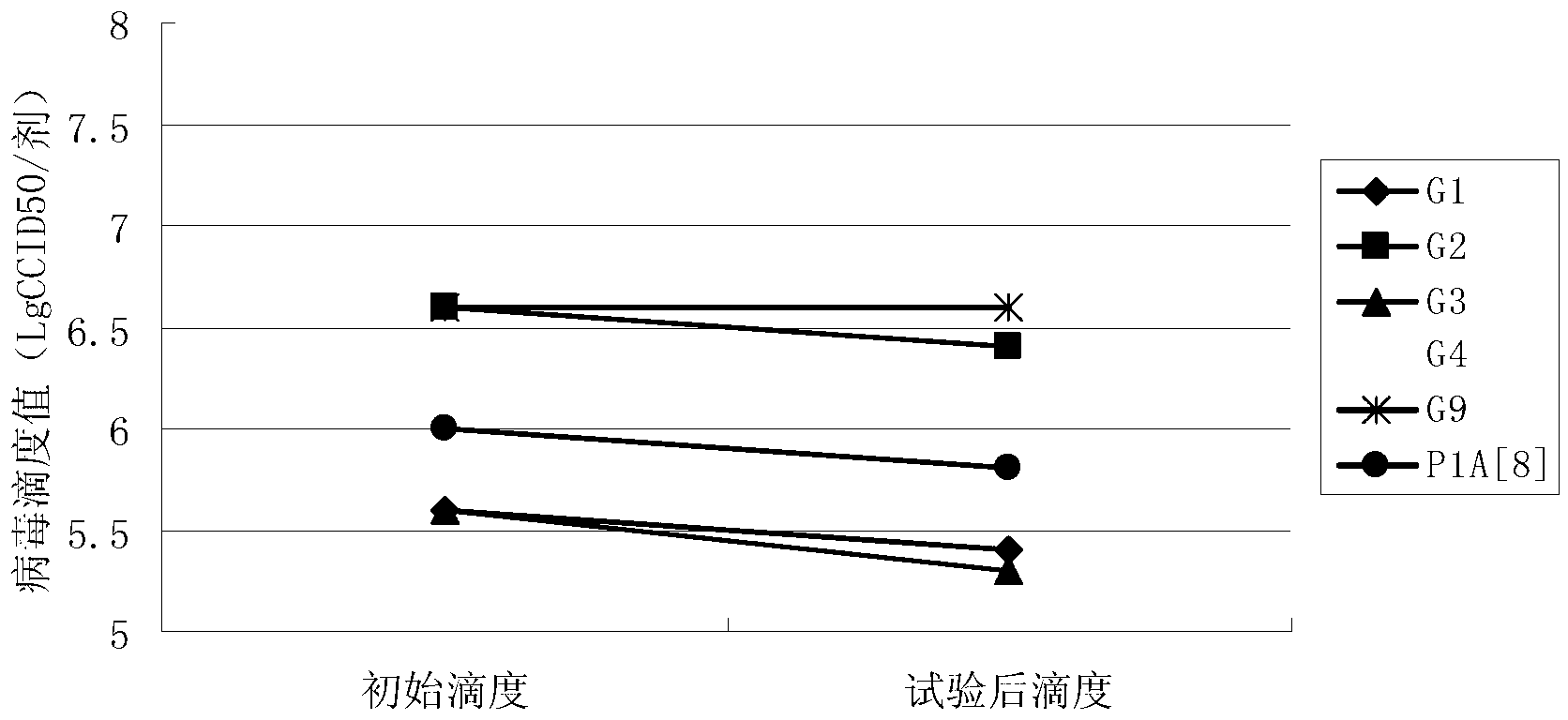
[0046] Embodiment 3 contains the antiacid effect of the rotavirus vaccine of different sucrose concentration formulations
[0047] (1) Preparation of vaccine preparations
[0048] Prepare 4 groups of vaccines containing different sucrose concentrations: Prepare 4 groups of 10ml 3M phosphate buffer solution (the molar concentration ratio of potassium dihydrogen phosphate and dipotassium hydrogen phosphate is 1:2) and 15ml 4M sodium succinate solution, and add to the 4 groups Sucrose solutions containing 30g, 90g, 150g, and 180g of sucrose were added to the mixed solution to prepare 4 kinds of protective agents containing different concentrations of sucrose (phosphate solution + sodium succinate solution + sucrose solution), and added to the 4 groups of protective agents The 100ml antigen component in Example 1 (same as Example 2, mixed with 6 serotypes) was filled up to 300ml with virus-free serum-free MEM maintenance solution, filtered and sterilized, and divided into 3ml dose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com