New gefitinib crystal form and preparation method thereof
A gefitinib and crystal form technology, applied in the field of new crystal forms of anticancer drugs and their preparation, can solve the problems of not mentioning which crystal form of gefitinib exists, not disclosing X-ray diffraction methods, etc. , to achieve the effect of cheap and easy-to-obtain reagents, mild conditions and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
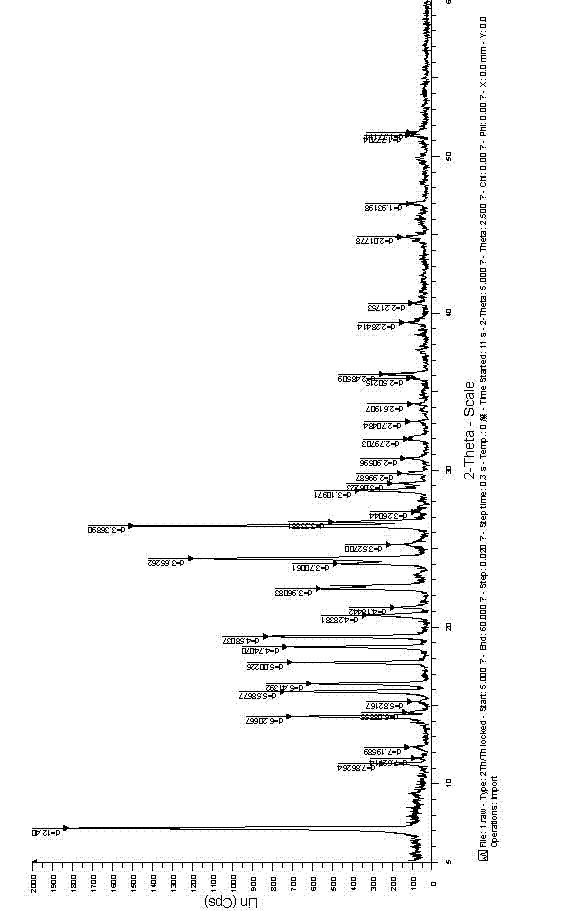
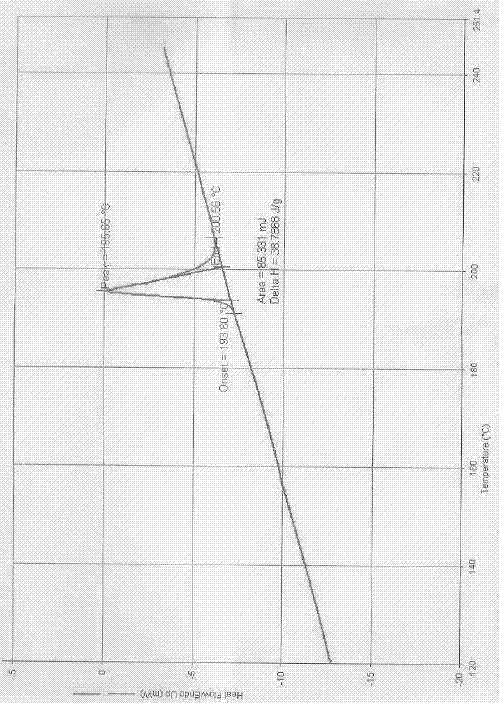
[0036] Add 10.0g of gefitinib and 100ml of DMF into the reactor, dissolve at room temperature (about 25°C) for 30min, add 150ml of tert-butyl methyl ether anti-solvent, the system begins to crystallize, and crystallize at room temperature for 6h, filter and dry to obtain off-white solid 8.2g, the product was confirmed to be gefitinib α crystal form by XRPD and DSC analysis, with a purity of 99.5% and a yield of 82%.
Embodiment 2
[0038] 200ml of chloroform was used instead of 100ml of DMF, and other operations were performed according to Example 1 to obtain 8.1g of off-white solid. The product was confirmed to be Gefitinib α crystal form by XRPD and DSC analysis, with a purity of 99.5% and a yield of 81%.
Embodiment 3
[0040] 200ml of acetonitrile was used instead of 100ml of DMF, and the rest were operated according to Example 1 to obtain 8.1g of off-white solid. The product was confirmed to be Gefitinib α crystal form by XRPD and DSC analysis, with a purity of 99.5% and a yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com