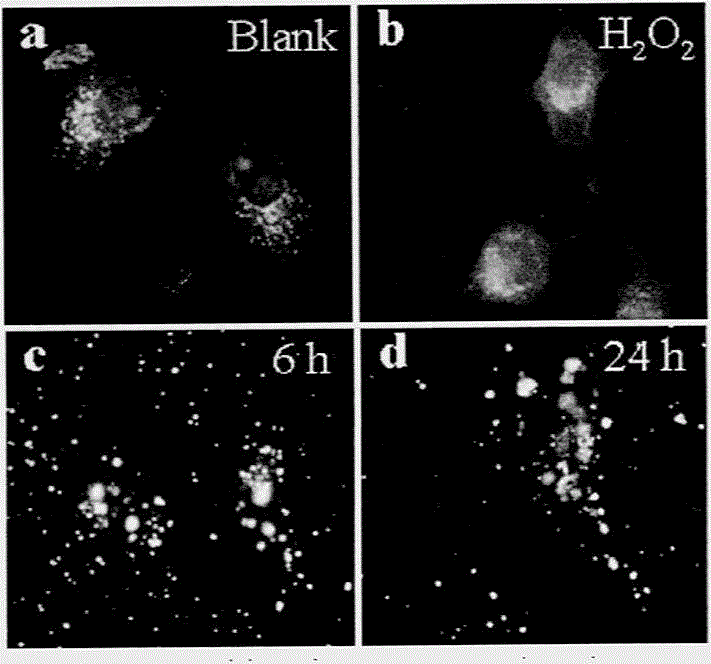
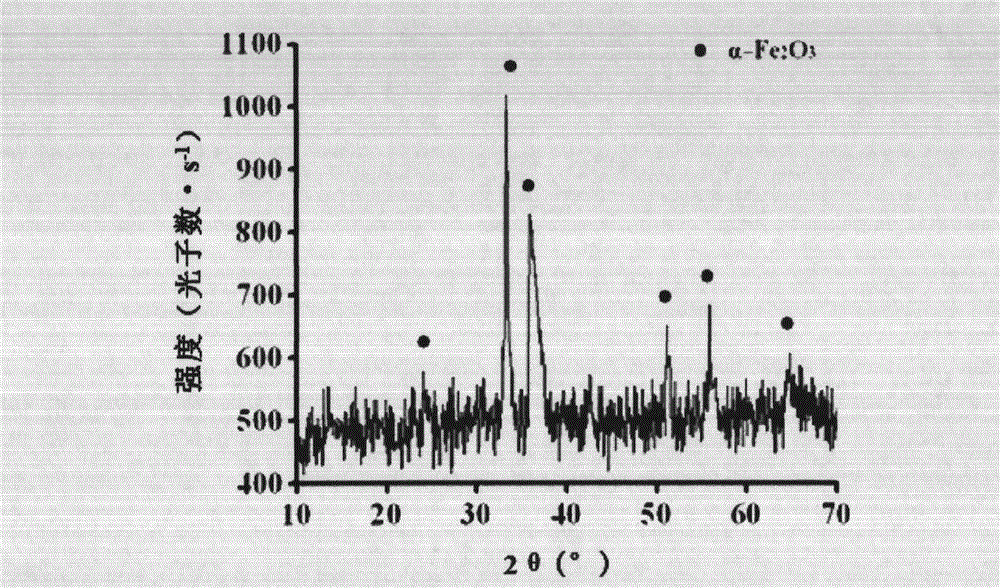
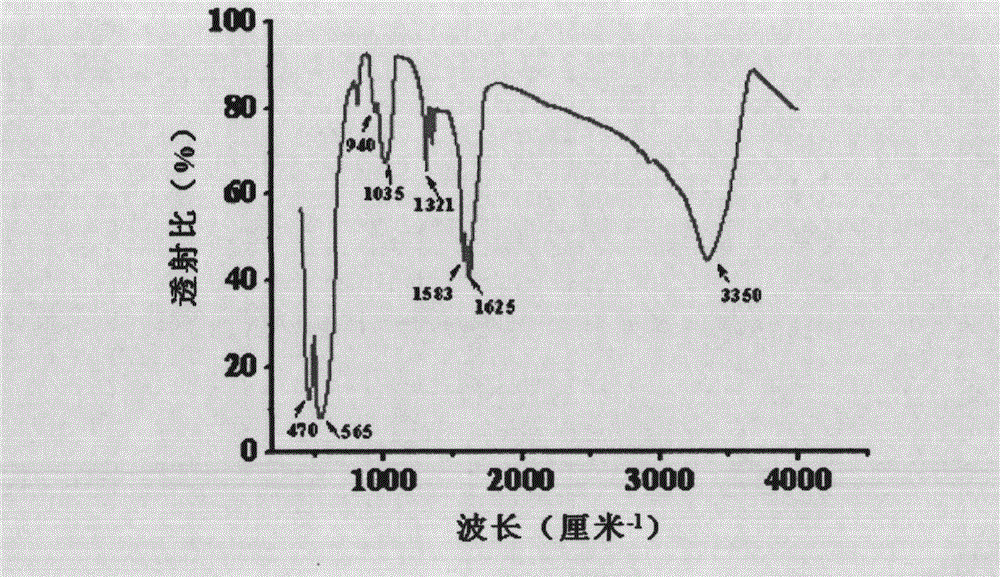
Method for preparing monodispersed alpha-Fe2O3 nanoparticles
A nanoparticle, monodisperse technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., to achieve the effect of excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Prepare 10mL0.4mol / L ferric nitrate solution: weigh 1.47g Fe(NO 3 ) 3 ·7H 2 O, dissolved in 10mL ultrapure water, shake at low speed (100rpm) to dissolve completely.
[0032] (2) Prepare 10 mL of 0.4 mol / L glycine solution: weigh 0.30 g of glycine, dissolve it in 10 mL of ultrapure water, and dissolve it completely under ultrasonic conditions.
[0033] (3) Transfer the solutions in (1) and (2) to a hydrothermal reaction kettle with a volume of 150 mL, and add 47 mL of ultrapure water and 33 mL of absolute ethanol. After buckling the lid of the reaction kettle, shake for 1 min to mix the solution evenly.
[0034] (4) The reaction kettle was placed in a temperature-programmed device, with a heating rate of 20 degrees per minute, and after the temperature was finally stabilized at 180 degrees, the reaction time was controlled to be 12 hours.
[0035] (5) Cool to room temperature after the reaction is complete, wash the obtained product three times with ethanol and ...
Embodiment 2
[0037] (1) Prepare 8mL0.2mol / L ferric nitrate solution: weigh 0.735g Fe(NO 3 ) 3 ·7H 2 O, dissolved in 8mL ultrapure water, shake at low speed (80rpm) to dissolve completely.
[0038] (2) Prepare 10 mL of 0.2 mol / L glycine solution: weigh 0.15 g of glycine, dissolve it in 10 mL of ultrapure water, and dissolve it completely under ultrasonic conditions.
[0039] (3) Transfer the solutions in (1) and (2) to a hydrothermal reaction kettle with a volume of 150 mL, and add 24 mL of ultrapure water and 16 mL of absolute ethanol. After buckling the lid of the reaction kettle, shake for 2 minutes to mix the solution evenly.
[0040] (4) The reaction kettle was placed in a temperature-programmed device, with a heating rate of 15 degrees per minute, and after the temperature was finally stabilized at 175 degrees, the reaction time was controlled to be 10 hours.
[0041] (5) Cool to room temperature after the reaction is complete, wash the obtained product three times with ethanol an...
Embodiment 3
[0043] (1) Prepare 12mL0.6mol / L ferric nitrate solution: weigh 2.646g Fe(NO 3 ) 3 ·7H 2 O, dissolved in 12mL ultrapure water, shake at low speed (120rpm) to dissolve completely.
[0044] (2) Prepare 10 mL of 0.6 mol / L glycine solution: weigh 0.45 g of glycine, dissolve it in 10 mL of ultrapure water, and dissolve it completely under ultrasonic conditions.
[0045] (3) Transfer the solutions in (1) and (2) to a hydrothermal reaction kettle with a volume of 150 mL, and add 72 mL of ultrapure water and 60 mL of absolute ethanol. After buckling the lid of the reaction kettle, shake for 3 minutes to mix the solution evenly.
[0046] (4) The reaction kettle was placed in a temperature-programmed device, and the temperature was finally stabilized at 185 degrees at a heating rate of 25 degrees per minute, and the reaction time was controlled to be 14 hours.
[0047] (5) After the reaction is complete, cool to room temperature, wash the obtained product three times with ethanol and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com