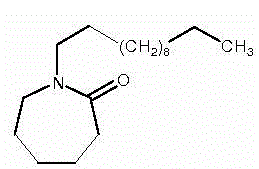
Production method of medicinal laurocapram
The invention relates to a technology of lauro azone and a production method, which are applied in the production of lauro azone and the production field of high-purity lauro azone, and can solve the problems of long production time, large amount of alkali used, etc., and achieve less environmental pollution and less reaction The effect of moderate temperature and simple production operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] Preparation of Solid Superbase Catalyst
[0030] Weigh KOH solid 28.1g, KF solid 29.1g, Al 2 o 3 Put 104.0g of fine powder into the reaction kettle, add 300mL of ethanol, turn on mechanical stirring, raise the temperature to 75°C, reflux for 1h, distill off the solvent under reduced pressure, put the solid in an oven, dry and activate at 115°C for 3h, and obtain KF-KOH / Al 2 o 3 Solid super base.
Embodiment 1
[0032] Weigh caprolactam 11.3g (0.1mol), KF-KOH / Al 2 o 3 Put 20.0g of catalyst into a stirred and heated reactor, add 100mL of cyclohexane solvent, raise the temperature to 45°C, stir for 1h, then add 27.4g of bromododecane, and 0.90g of KI solid, heat up to 70~75°C and reflux Reaction 1h. The reaction solution was filtered, and the filter residue was washed with an appropriate amount of cyclohexane until the identification reaction of laurocapram did not appear. The filtrate and cleaning solution were combined, and washed with saturated saline until the washing solution was close to neutral. After evaporating the solvent and rectifying under reduced pressure, collecting the fraction at 158-160°C under a vacuum of 0.66kPa to obtain 27.2 g of laurocapram, the content of laurocapram was >99.5%, and the yield was 93.9%.
Embodiment 2
[0034] Weigh caprolactam 56.5g (0.5mol), KF-KOH / Al 2 o 3 Put 100.0g of catalyst into a stirred and heated reactor, add 500mL of cyclohexane solvent, raise the temperature to 45°C, stir for 1.5h, then add 137.1g of bromododecane, and 4.5g of KI solid, and raise the temperature to 70~75°C Reflux reaction for 1.5h. The reaction solution was filtered, and the filter residue was washed with an appropriate amount of cyclohexane until the identification reaction of laurocapram did not appear. The filtrate and cleaning solution were combined, and washed with saturated saline until the washing solution was close to neutral. After evaporating the solvent and rectifying under reduced pressure, collecting the fraction at 158-160°C under a vacuum of 0.66kPa to obtain 129.6g of laurocaprazine, the content of laurocaprazine was >99.5%, and the yield was 94.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com