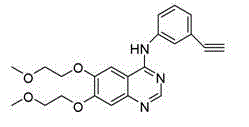
Preparation method of hydrochloric acid erlotinib crystal form F
A technology for erlotinib hydrochloride and crystal form, which is applied in the field of preparing erlotinib hydrochloride crystal form F, can solve the problem that it is not suitable for highly flammable solvents, is not suitable for industrial-scale preparation, and is not suitable for erlotinib hydrochloride crystal form F. No reproducibility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0053] The method of the present invention is illustrated in more detail by the following non-limiting examples. The following examples do not limit the invention in any way.
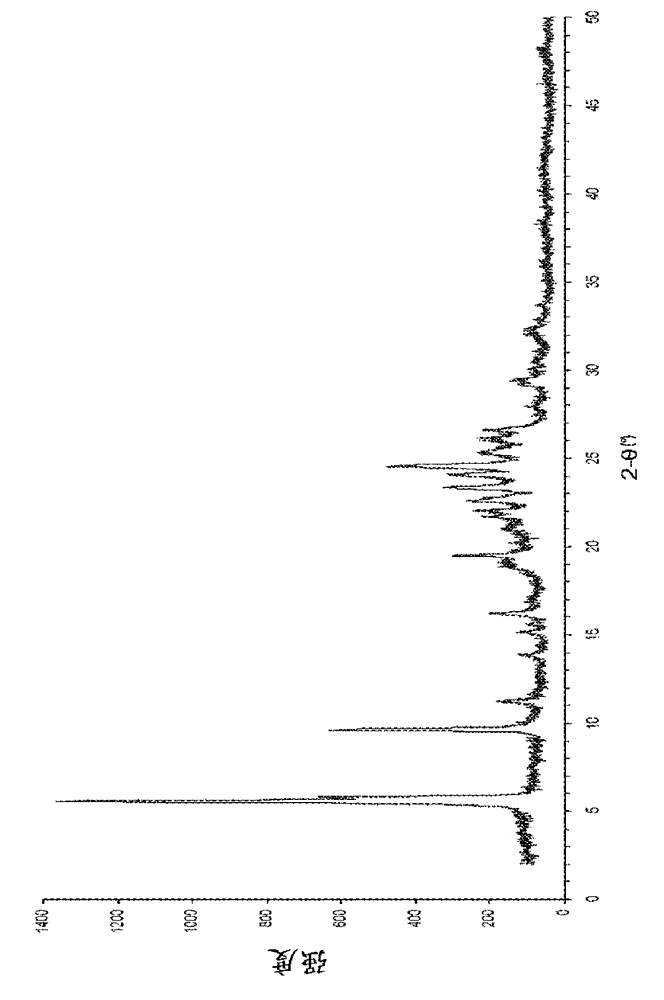
[0054] In the following comparative examples and examples, X-ray powder diffraction analysis was performed under the following conditions:
[0055] The X-ray powder diffraction pattern was obtained using a PANalytical-X’Pert diffractometer (using CuKα-radiation). The system is configured for θ-θ, transmission geometry and installed with a 3152 / 63 focusing X-ray mirror, 0.5o divergence and anti-scatter slits, and 0.02 rad incident soller slit . Load the sample on the PW3064 / 60 reflection / transmission rotating stage. The detector is a 3018 / 00 PIXcel detector, equipped with a 2 mm anti-scatter slit for transmission and a 0.02 rad Soler slit. Use a standard sample holder to load approximately 10-20 mg of sample between the two transparent films. The X'Pert data collector (version 2.2g) was used to collect d...
Embodiment 1~4
[0066] Erlotinib free base (1.0-1.2 g) was added to the solvent shown in Table 2 below (the corresponding amount is shown in Table 2), heated and dissolved at 80-100°C. At 30°C, the resulting solution was added to a mechanically stirred solution of hydrogen chloride (0.55~0.62 mL, 5.7M isopropanol, 1.2 molar equivalent) in the solvent (5 mL) shown in Table 2 within 30 minutes. The mixture was stirred at 30°C for 1 hour, filtered and washed with the solvent shown in Table 2 below (2 mL). The collected solid was vacuum dried at 50°C. The product was analyzed by powder X-ray diffraction and determined to be crystal form F.
[0067] Table 2
[0068] Example No. Solvent Volume (mL) Yield Crystal form 1 1-butanol10 1.01g (90%)F 2 2-butanol10 0.99g (88%)F 3 Tert-butanol15 0.97g (87%)F 4 2-methylpropanol10 1.00 g (82%) F
Embodiment 5
[0070] Erlotinib free base (10 g) was added to 2-methylpropanol (100 mL), heated at 70°C and dissolved. At 30°C, the resulting solution was added to mechanically stirred hydrogen chloride (4.7 mL, 6M isopropanol, 1.1 molar equivalent) in isopropyl acetate (50 mL) and 2-methylpropanol (50 mL) within 15 minutes. mL) of the mixed solution. The mixture was stirred at 30°C for 30 minutes, filtered and washed with 2-methylpropanol (20 mL). The collected solid was vacuum dried at 60°C. The product was analyzed by powder X-ray diffraction and determined to be crystal form F. Yield: 9.55g (87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com