Preparation method of positive electrode catalyst Mn2O3 of lithium carbon dioxide battery
A carbon dioxide, battery cathode technology, applied in battery electrodes, fuel cell type half cells and secondary battery type half cells, circuits, etc., can solve the problems of high cost, short cycle life, unsuitable promotion, etc. Low cost, simple operation, good cycle performance and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A kind of cathode catalyst Mn of lithium carbon dioxide battery 2 o 3 The preparation method, the steps are as follows:
[0024] 1) Dissolve 0.5g of PVP (polyvinylpyrrolidone) in 50ml of absolute ethanol to obtain mixed solution 1.
[0025] 2) Add 1g Mn(CH 3 COO) 2 4H 2 O (manganese acetate tetrahydrate) was dissolved in mixed solution 1 to obtain mixed solution 2.
[0026] 3) The above mixed solution 2 was magnetically stirred at 50°C and refluxed for 3 hours; after the reaction was completed, it was naturally cooled to room temperature to obtain a white precipitated product; Vacuum dried for 12 h to obtain a powdery precursor.
[0027] 4) Place 0.8 g of the precursor obtained above in a tube furnace in an air atmosphere and heat it to 500 °C at a heating rate of 0.5 °C / min, and keep it warm for 4 hours to obtain a porous lithium-carbon dioxide battery cathode catalyst material Mn 2 o 3 .
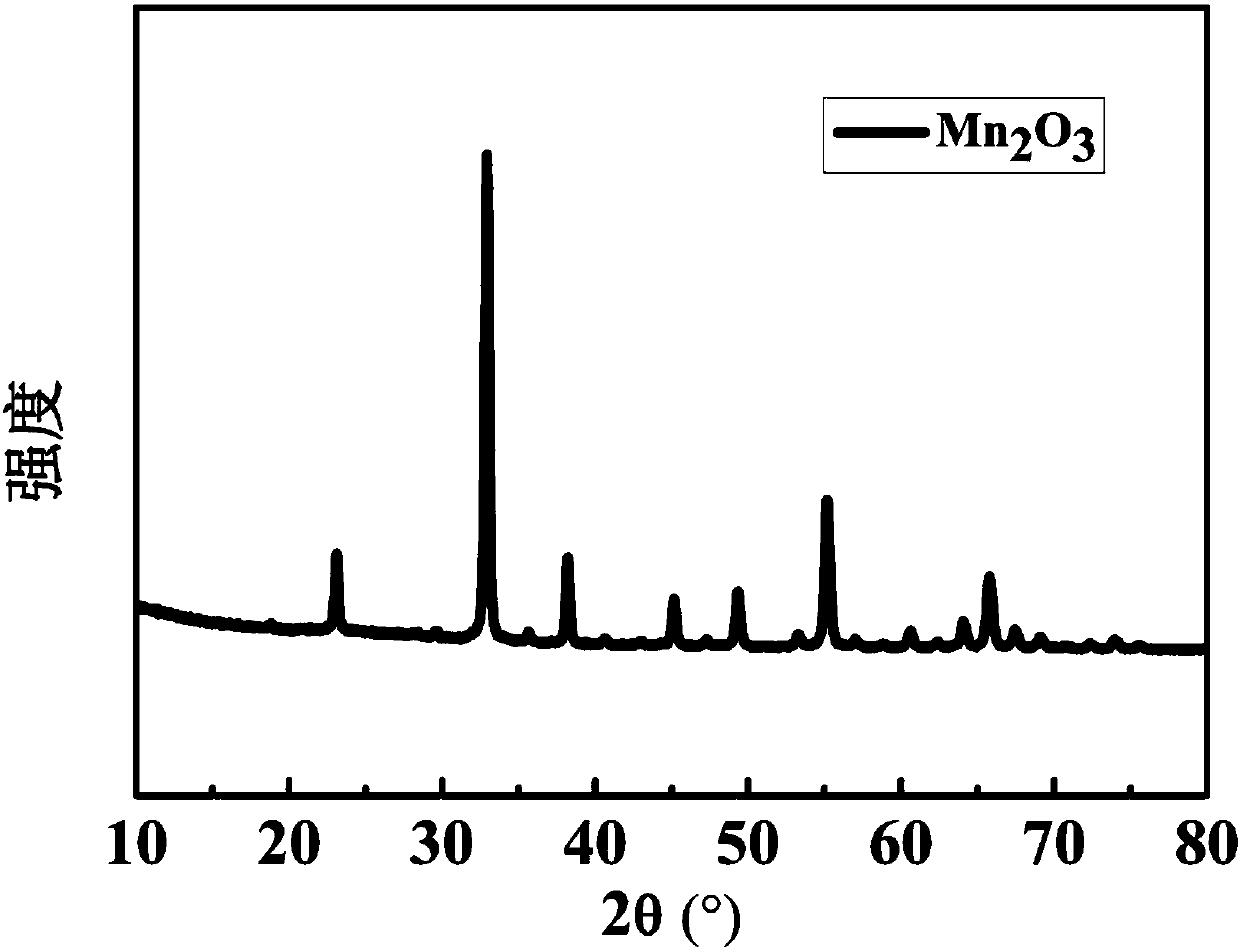
[0028] figure 1 for Mn 2 o 3 XRD (X-ray Diffraction) diffraction pa...
Embodiment 2
[0034] A kind of cathode catalyst Mn of lithium carbon dioxide battery 2 o 3 The preparation method, the steps are as follows:
[0035] 1) Dissolve 0.6g of PVP (polyvinylpyrrolidone) in 60ml of absolute ethanol to obtain mixed solution 1.
[0036] 2) Add 1.2g Mn(CH 3 COO) 2 4H 2 O (manganese acetate tetrahydrate) was dissolved in mixed solution 1 to obtain mixed solution 2.
[0037] 3) The above mixed solution 2 was magnetically stirred at 50°C and refluxed for 3 hours; after the reaction was completed, it was naturally cooled to room temperature to obtain a white precipitated product; Vacuum dried for 12 h to obtain a powdery precursor.
[0038] 4) Place 0.9 g of the precursor obtained above in a tube furnace in an air atmosphere and heat it to 600 °C at a heating rate of 0.5 °C / min, and keep it warm for 3 hours to obtain a porous lithium carbon dioxide battery cathode catalyst material Mn 2 o 3 .
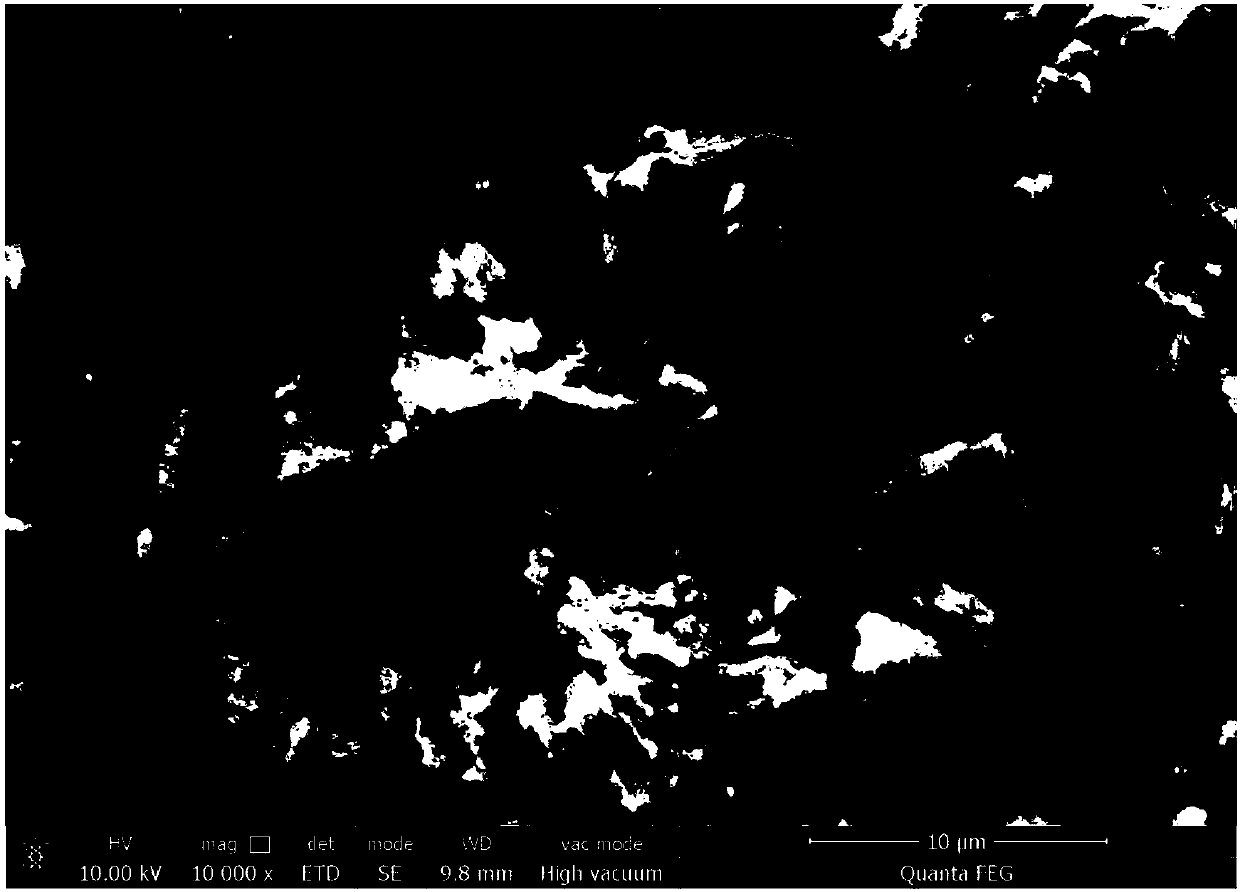
[0039] The obtained Mn 2 o 3 The XRD diffractogram and SEM scannin...
Embodiment 3
[0044] A kind of cathode catalyst Mn of lithium carbon dioxide battery 2 o 3 The preparation method, the steps are as follows:
[0045] 1) Dissolve 0.8g of PVP (polyvinylpyrrolidone) in 80ml of absolute ethanol to obtain mixed solution 1.
[0046] 2) Add 1.5g Mn(CH 3 COO) 2 4H 2 O (manganese acetate tetrahydrate) was dissolved in mixed solution 1 to obtain mixed solution 2.
[0047] 3) The above mixed solution 2 was magnetically stirred at 50°C and refluxed for 3 hours; after the reaction was completed, it was naturally cooled to room temperature to obtain a white precipitated product; Vacuum dried for 12 h to obtain a powdery precursor.
[0048] 4) Place 0.8 g of the precursor obtained above in a tube furnace in an air atmosphere and heat it to 600 °C at a heating rate of 1 °C / min, and keep it warm for 3 hours to obtain a porous lithium-carbon dioxide battery cathode catalyst material Mn 2 o 3 .
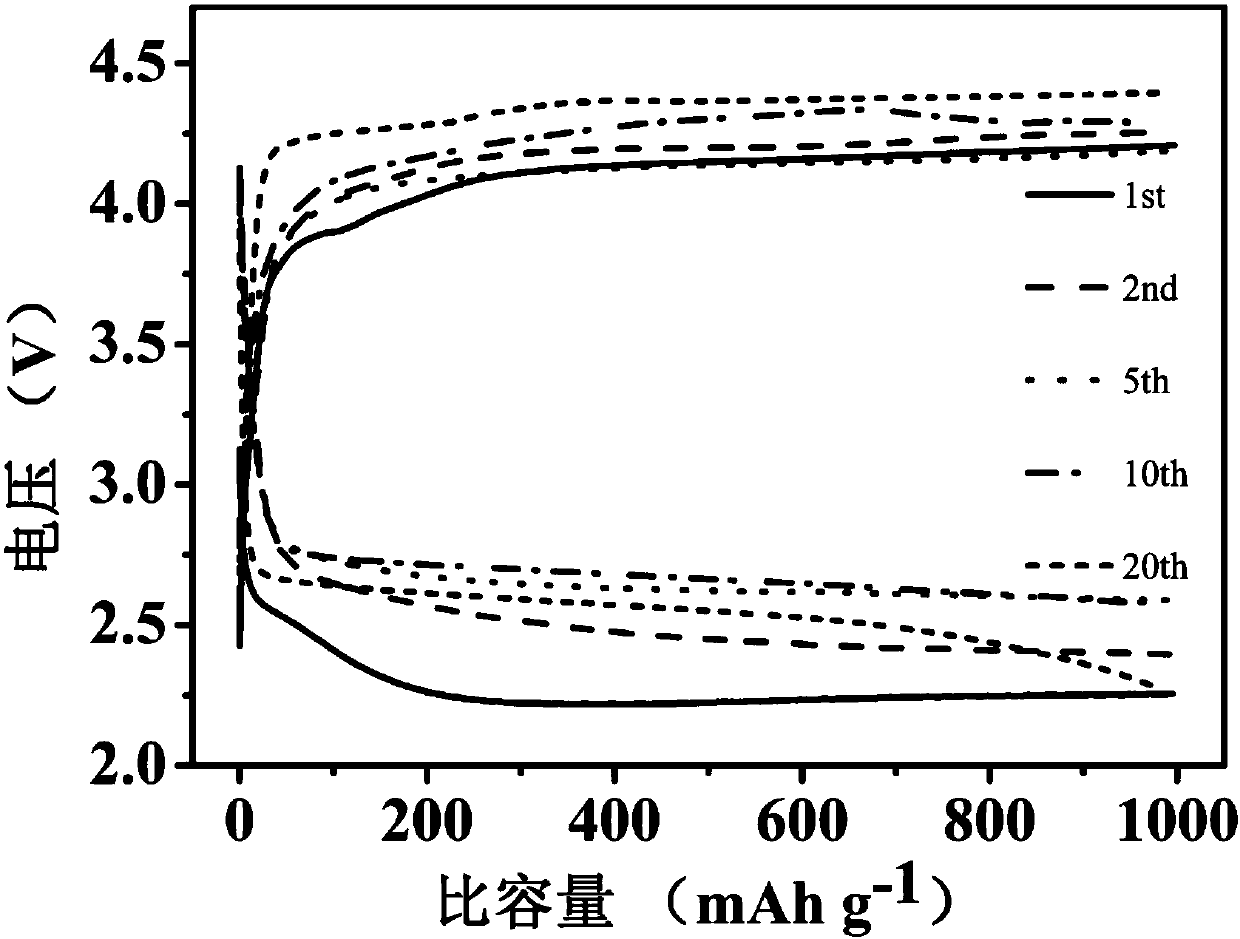
[0049] The obtained Mn 2 o 3 The XRD diffractogram and SEM scanning ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com