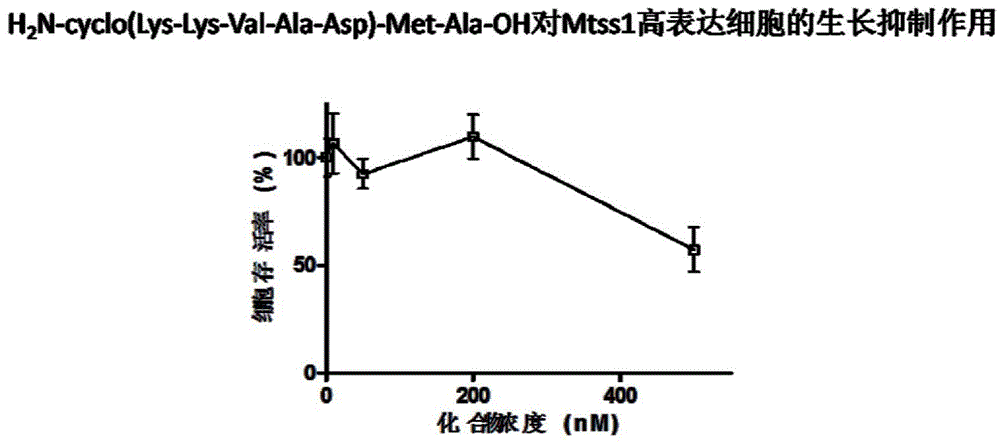
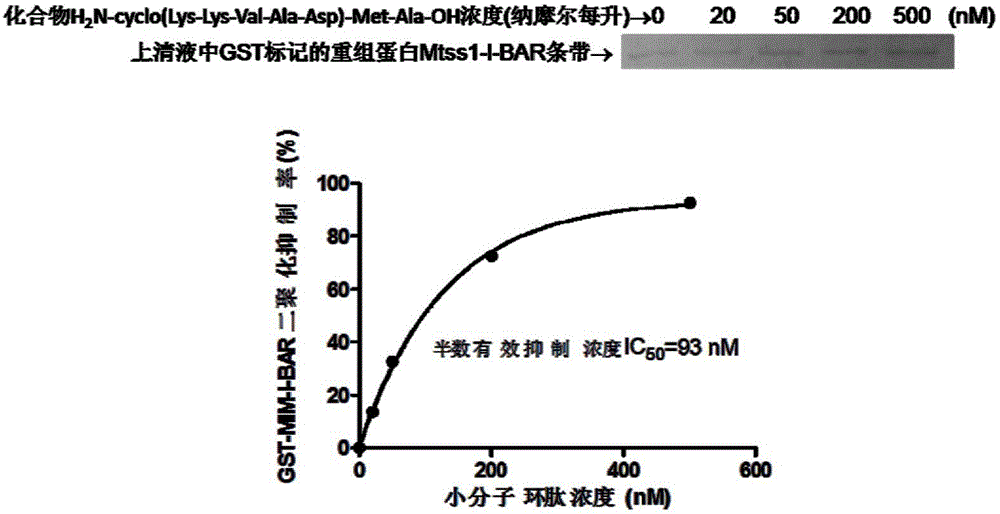
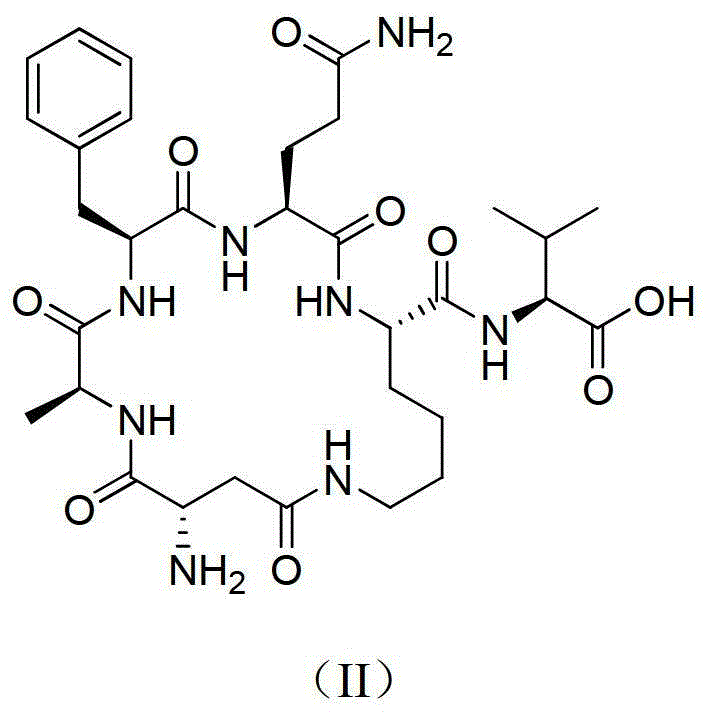
Metastatic tumor deletion protein small-molecule cyclopeptide inhibitor as well as preparation method and application thereof
A technology for metastatic tumors and small molecules, applied in the field of biochemistry, can solve the problems of short peptides that are difficult to obtain inhibitory effects, difficult to ensure stability, and large molecular weight of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H2
[0100] Example 1H 2 Preparation of N-cyclo(Asp-Ala-Phe-Gln-Lys)-Val-OH
[0101] 0.8g of Fmoc-Val-OBzl- The resin was soaked in 10ml of anhydrous DMF and stirred at room temperature for 30min. Afterwards, use 25% Pip in DMF to mix with the resin for 20 min to remove the Fmoc protecting group.
[0102] Use DMF to wash the resin, then add 3ml of 3.8g Fmoc-Lys(Boc)-OH in DMF, 3ml of 0.3mol / L DCC in DMF and 3ml of 0.4mol / L HOBt in DMF, stir at room temperature for 15min, and perform coupling reaction.
[0103] After the reaction finishes, use DMF to wash the resin, and use the DMF solution of 25%Pip to mix with the resin for 20min, to remove the Fmoc protecting group, then wash with DMF to obtain Fmoc-Lys(Boc)-Val-OBzl- Prepare for the next coupling reaction.
[0104]Use Fmoc-Gln-OH, Fmoc-Phe-OH, Fmoc-Ala-OH and Fmoc-Asp(OtBu)-OH cycles to carry out chain reaction until Fmoc-Asp(OtBu)-Ala-Phe-Gln-Lys is obtained (Boc)-Val-OBzl-
[0105] Use 0.1N HBr / HOAc to treat for 1 h...
Embodiment 2H2
[0107] Example 2H 2 Preparation of N-cyclo(Lys-Lys-Val-Ala-Asp)-Met-Ala-OH.
[0108] 1 mmol of Fmoc-Ala-OBzl- The resin was soaked in 10ml of anhydrous DMF and stirred at room temperature for 30min. Afterwards, use 10% Pip in DMF to mix with the resin for 30 min to remove the Fmoc protecting group.
[0109] Wash the resin with DMF, then add a DMF solution containing 2 mmol Fmoc-Met(Boc)-OH, 5 ml 0.3 mol / L DCC in DMF and 5 ml 0.4 mol / L HOBt in DMF, and stir at room temperature for 10 min to carry out the coupling reaction.
[0110] After the reaction, use DMF to wash the resin, and use the DMF solution of 3ml of 10%Pip to mix with the resin for 30min to remove the Fmoc protecting group, and then wash with DMF to obtain Fmoc-Met(Boc)-Ala-OBzl- , ready for the next coupling reaction.
[0111] Use Fmoc-Asp-OH, Fmoc-Ala-OH, Fmoc-Val-OH, Fmoc-Lys-OH and Fmoc-Lys(OtBu)-OH in sequence for chain coupling reaction until Fmoc-Lys(OtBu)-Lys is obtained -Val-Ala-Asp-Met-Ala--
[...
Embodiment 3
[0114] Example 3H 2 Preparation of N-cyclo(Lys-Ile-Gly-Ser-Asp)-Leu-OH.
[0115] 1 mmol of Fmoc-Leu-OBzl- The resin was soaked in 10ml of anhydrous DMF and stirred at room temperature for 30min. After that, use 30% Pip in DMF solution to mix with the resin for 10 min to remove the Fmoc protecting group.
[0116] Wash the resin with DMF, then add a DMF solution containing 3 mmol Fmoc-Asp(Boc)-OH, 5 ml 0.5 mol / L DCC in DMF and 5 ml 0.6 mol / L HOBt in DMF, and stir at room temperature for 20 min to carry out the coupling reaction.
[0117] After the reaction, use DMF to wash the resin, and use the DMF solution of 1ml of 30%Pip to mix with the resin for 10min to remove the Fmoc protecting group, and then wash with DMF to obtain Fmoc-Met(Boc)-Ala-OBzl- Prepare for the next coupling reaction.
[0118] Use Fmoc-Ser-OH, Fmoc-Gly-OH, Fmoc-Ile-OH, Fmoc-Lys-OH cycle to carry out chain reaction until Fmoc-Lys(OtBu)-Ile-Gly-Ser-Asp-
[0119] Use 0.12N HBr / HOAc to treat for 0.5h to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com