Novel purpose of microRNA-30 family
A family and functional technology, applied in the new field of application of the microRNA-30 family, can solve problems such as unclear proteinuria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
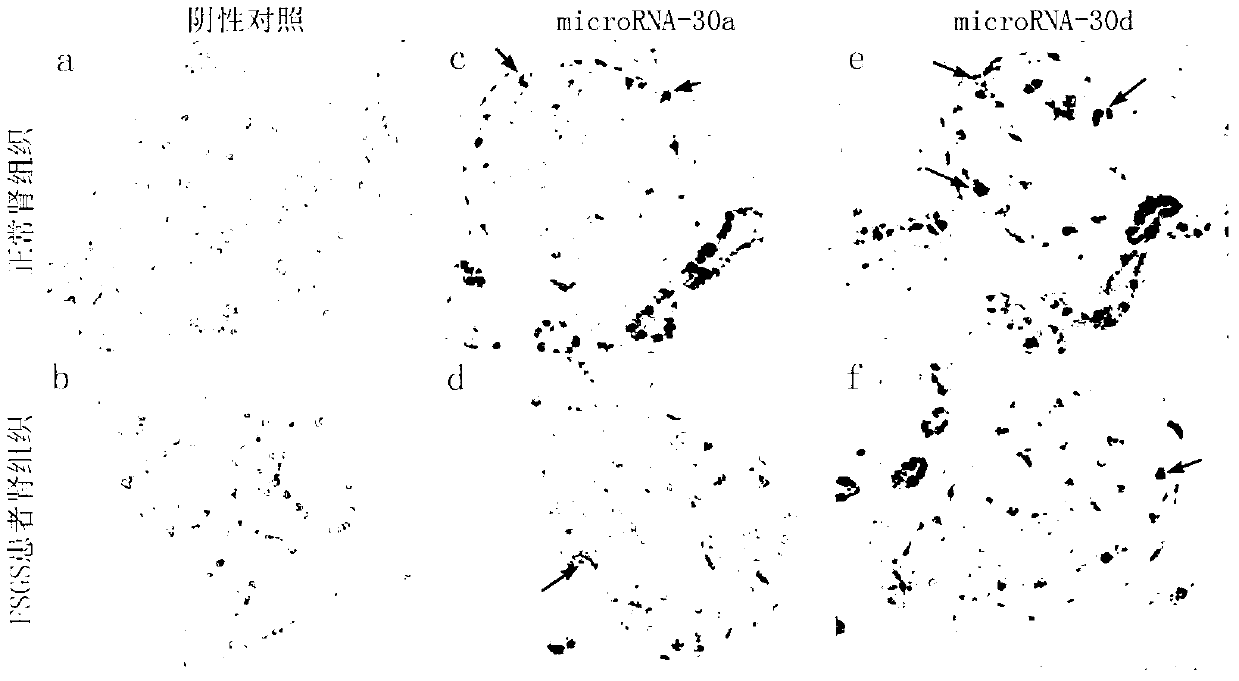
[0031] Example 1 Application of microRNA30 family as podocyte injury markers in patients with focal segmental glomerulosclerosis (FSGS)
[0032] The microRNA-30 family is one of the important components of microRNAs. The human and mouse microRNA-30 families include five members: microRNA-30a, microRNA-30b, microRNA-30c, microRNA-30d, and microRNA-30e. The five members of the microRNA-30 family are all expressed in podocytes (human, rat, mouse) and are abundant. In the application of the microRNA30 family as a marker of podocyte injury in patients with focal segmental glomerulosclerosis (FSGS), the specific detection methods are:
[0033] (1) In situ hybridization
[0034] Kidney tissue paraffin specimens were freshly prepared for xylene dewaxing, dehydrated with graded alcohol, and freshly prepared with 0.5% H 2 o 2 Treat at room temperature for 30 minutes to inactivate endogenous peroxidase; freshly prepare protease (0.01g / L) with 3% citric acid, and treat at 37°C for 10 m...
Embodiment 2
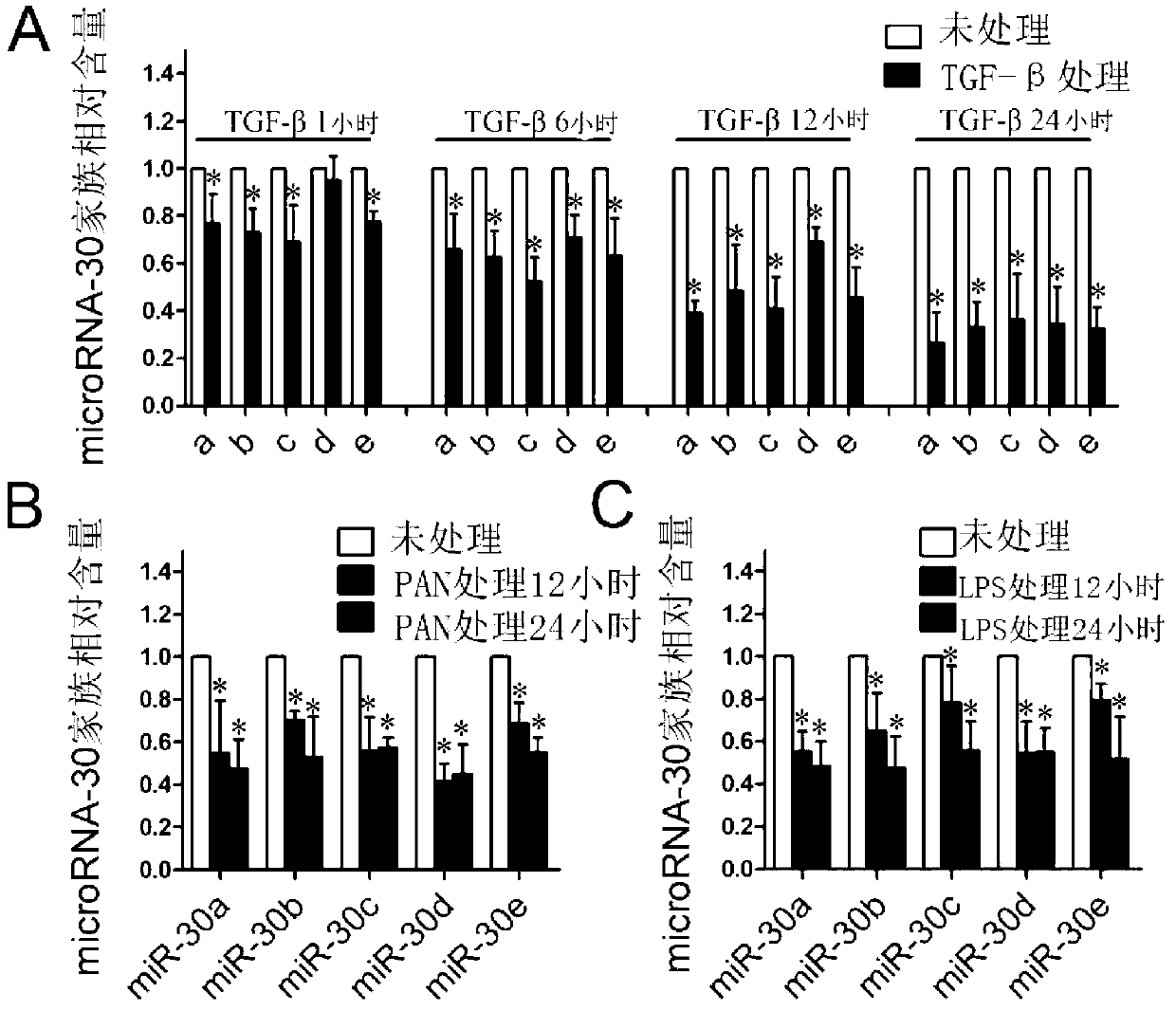
[0038] Example 2 The new application of the microRNA-30 family in the preparation of podocyte therapeutic drugs
[0039] (1) Plasmid transfection method
[0040] 1) MicroRNA-30a plasmid construction: ① Gene sequence synthesis: use pSilencer2.0U6 plasmid as a carrier, synthesize microRNA-30a sequence by PCR method, and add BamHI restriction site (GATCC) at the 5' end, and add HindⅢ ( A) Restriction site, and add 5 Ts as the terminator of RNA polymerase III, the final synthesized sequence is
[0041] gatccgcgactgtaaacatcctcgactggaagctgtgaagccacagatgggctttcagtcggatgtttgcagctgcttttta and simultaneously synthesized microRNA30a reverse complementary pairing sequence gtaaaaagcagctgcaaacatccgactgaaagcccatctgtggcttcacagcttccagtcgaggatgtttacagtcgcggatcttcga. ②Use a PCR instrument to anneal and ligate the above-mentioned single-stranded oligonucleotide fragments; ③The vector pSilencer2.0U6 plasmid is linearized and recovered: add the vector pSilencer2.0U6 plasmid (10 μl), BamHI enzyme (...
Embodiment 3
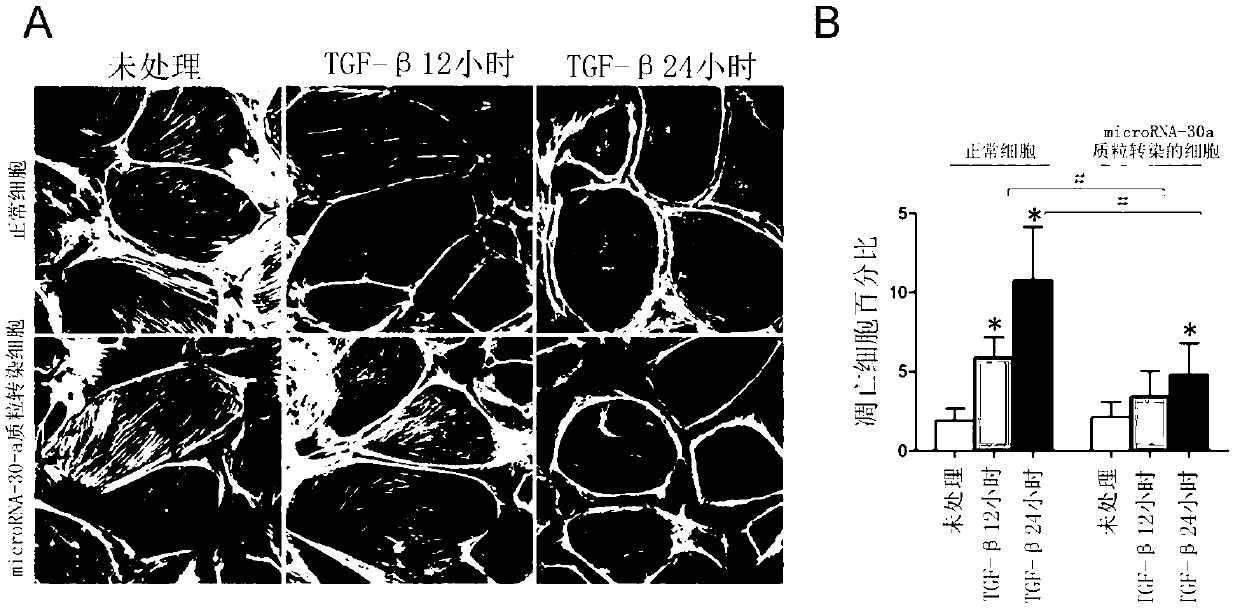
[0049] Embodiment three plasmid animal experiments
[0050] Injection method of microRNA-30a plasmid in rat tail vein:
[0051] (1) MicroRNA-30a plasmid preparation (the method is the same as the plasmid transfection method in Example 2).
[0052] (2) Tail vein injection of microRNA-30a plasmid: the specific operation is as follows. Wistar rats were anesthetized without anesthesia or 10% chloral hydrate (dose 3ml / Kg), and injected 80ml / kg into the tail vein with a 24G intravenous trocar Ringer's solution (containing 100 μg plasmid encoding microRNA-30a gene), the injection was completed within 10 seconds.
[0053] The result is as Figure 4 As shown, the microRNA-30a plasmid treatment can significantly reduce the 24-hour proteinuria of PAN rats, and the proteinuria of the rats was significantly increased by 117±53mg on the 5th day, 7th day, and 9th day after PAN (5mg / 100g) injection / 24h, 182±75, 235±60, after microRNA-30a plasmid tail vein injection on the 5th day, 7th day...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com