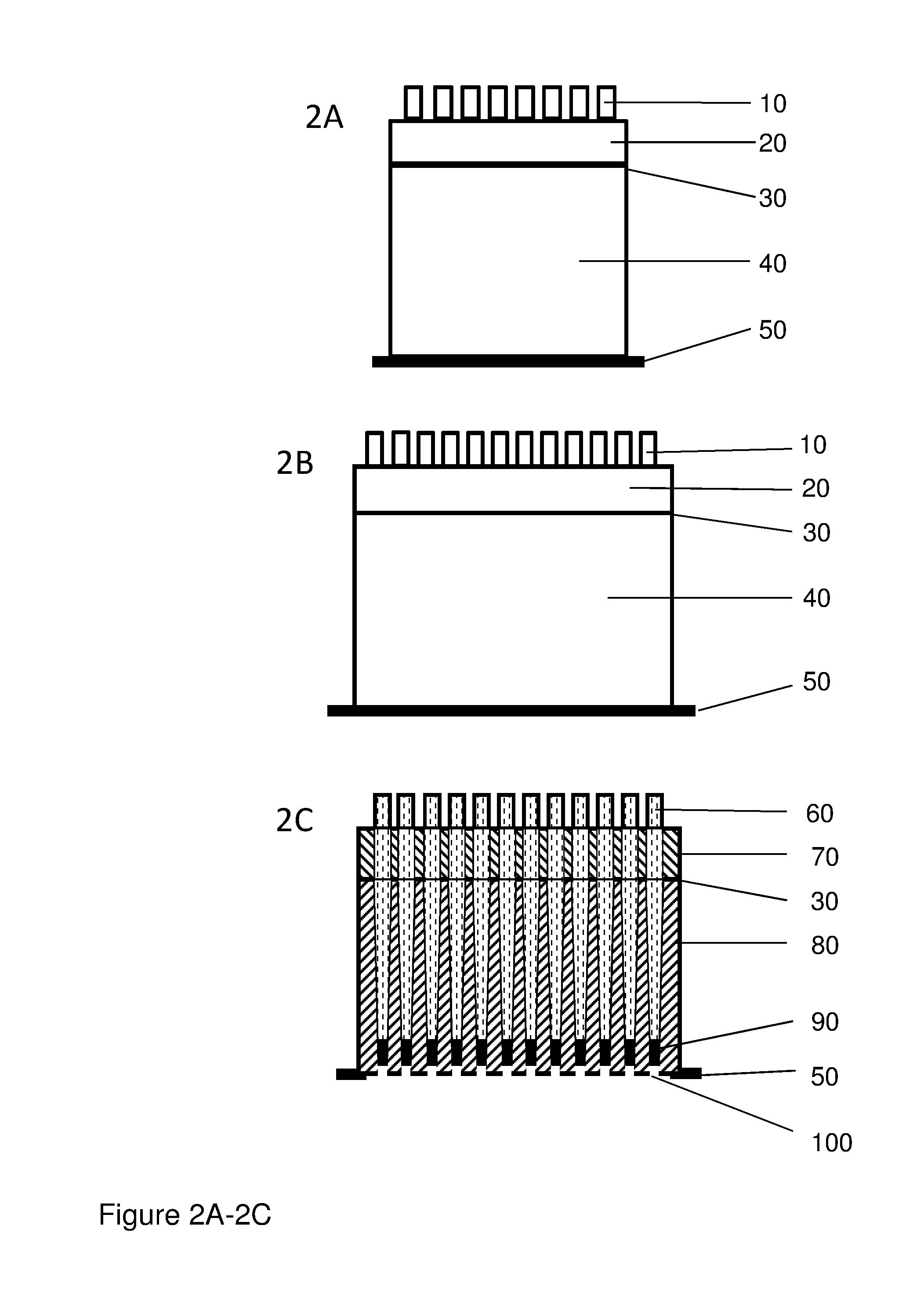
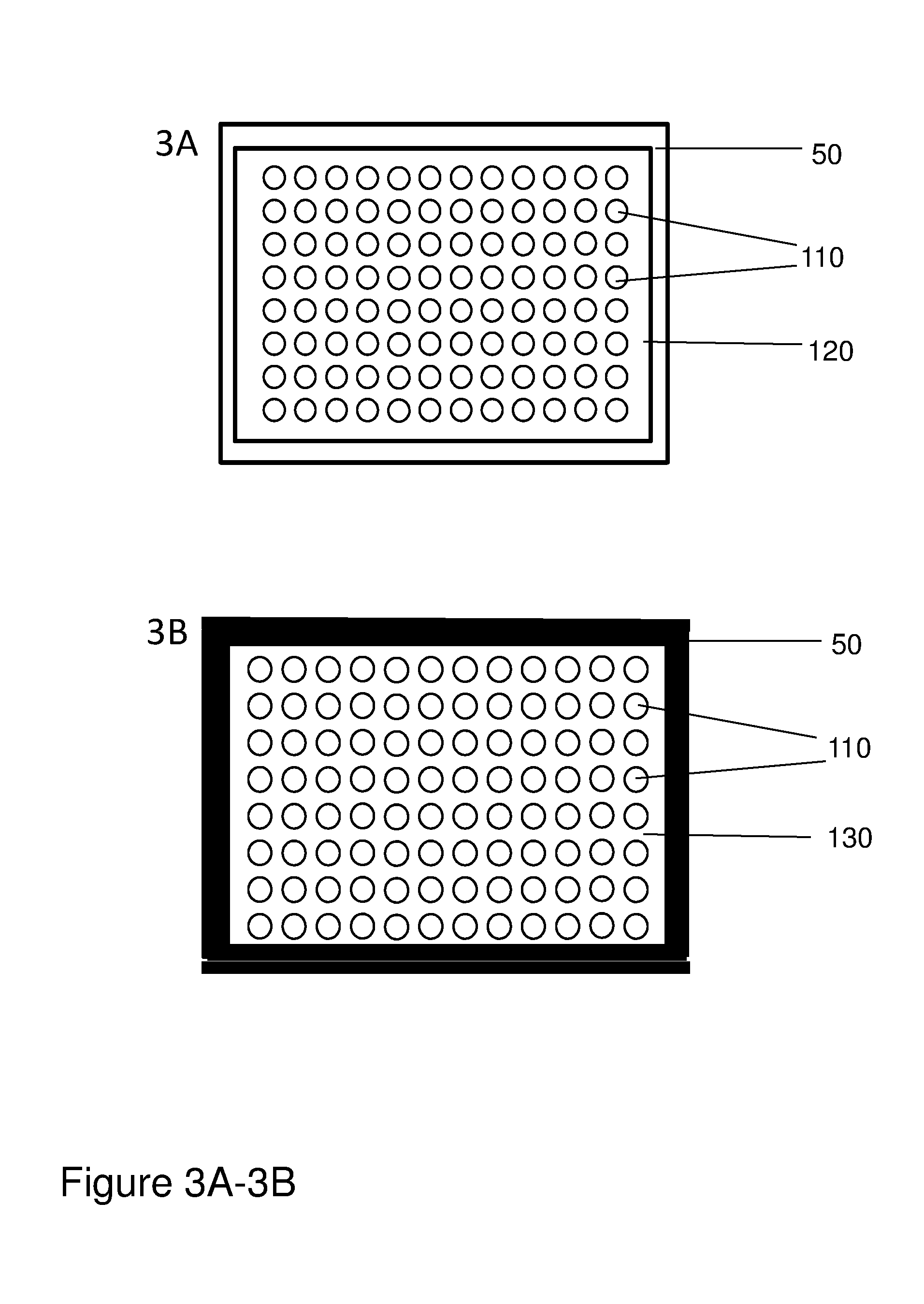
Patents
Literature
55 results about "Plasmid preparation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A plasmid preparation is a method of DNA extraction and purification for plasmid DNA. Many methods have been developed to purify plasmid DNA from bacteria.
Method for controlling plasmid replication in escherichia coli
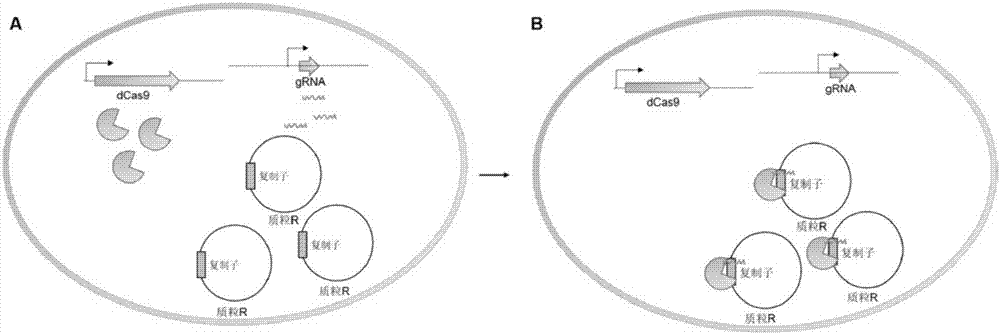
The invention discloses a method for controlling plasmid replication in escherichia coli. A plasmid control method developed is based on a Crispr / Cas9 system; plasmid replication is effectively regulated and controlled by the aid of expressed dCas9 protein and transcribed gRNA targeting a target sequence of a plasmid replication area, and the method is a brand new method which is not reported in other documents and patents. The target sequence targeting the replication area can have multiple choices, and the purpose of controlling the plasmid replication of different degrees is assuredly realized by the aid of different dCas9 / gRNA pairs. The method can effectively reduce the yield of plasmids by 2-34 times, and compared with a previous method reported in the patents, the method has the advantages of wide range of control and higher control capacity; in conclusion, the method is novel in design and can realize control over the plasmid replication of different degrees effectively.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
Recombinant plasmid for acetone-butanol clostridium gene disruption
InactiveCN101328486AIncrease insertion efficiencyImprove the imperfect situationVector-based foreign material introductionPlasmid VectorGenetics manipulation
The invention provides a recombinant plasmid, a preparation method thereof, an application, two knockout recombinant plasmid vectors pSY6-buk and pSY6-solR which are prepared by taking the recombinant plasmid as a starting plasmid, a method for preparing the recombiant plasmid vectors pSY6-buk and pSY6-solR, and an application. The recombinant plasmid can be taken as a knonckout starting plasmid of an acetone and butanol, can perform the gene knockout efficiently and rapidly, and can be evolved into a novel genetic manipulation system in order to improve the imperfect condition of the prior manipulation system in the current research on the clostridium acetobutylicum, thereby providing convenience for the research on the metabolic engineering of the clostridium acetobutylicum. The recombinant plasmid vectors pSY6-buk and pSY6-solR can be directly applied to establishing knockout strains of the clostridium acetobutylicum buk and solR genes.
Owner:SHANGHAI INST OF BIOLOGICAL SCI CHINESE ACAD OF SCI
Grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine
InactiveCN107760716AInducible propertiesImproving immunogenicityViral antigen ingredientsAntiviralsEscherichia coliDisease
The invention discloses a grass carp reovirus S11 gene eukaryotic expression recombinant plasmid preparation method and application thereof in serving as nucleic acid vaccine, and belongs to the technical field of gene engineering and molecular immunology. The preparation method disclosed by the invention is characterized by comprising the steps of extracting viral genome RNA, performing reverse transcription to convert the viral genome RNA into cDNA, amplifying a corresponding DNA sequence out, constructing the DNA sequence to pcDNA-3.1(+) plasmid, converting Escherichia coli DH5alpha, screening out positive clone bacteria containing recombinant plasmid, culturing a lot of the positive bacteria and extracting recombinant plasmid S11-pcDNA3.1 contained in the bacteria. The recombinant plasmid is utilized as nucleic acid vaccine to perform intramuscular injection on the grass carps, and the nucleic acid vaccine enters the muscle cells and expresses VP35 protein of the grass carp reovirus in the muscle cells; thus, fish body immune cell proliferation is stimulated, antiviral related gene expression is up regulated, fish bodies are stimulated to generate antiviral antibodies, and capability of grass carps in resisting grass carp reovirus infection is effectively improved; furthermore, the grass carp reovirus S11 gene eukaryotic expression recombinant plasmid can be used for preventing a grass carp hemorragic disease caused by the grass carp reovirus in aquaculture.
Owner:HENAN NORMAL UNIV
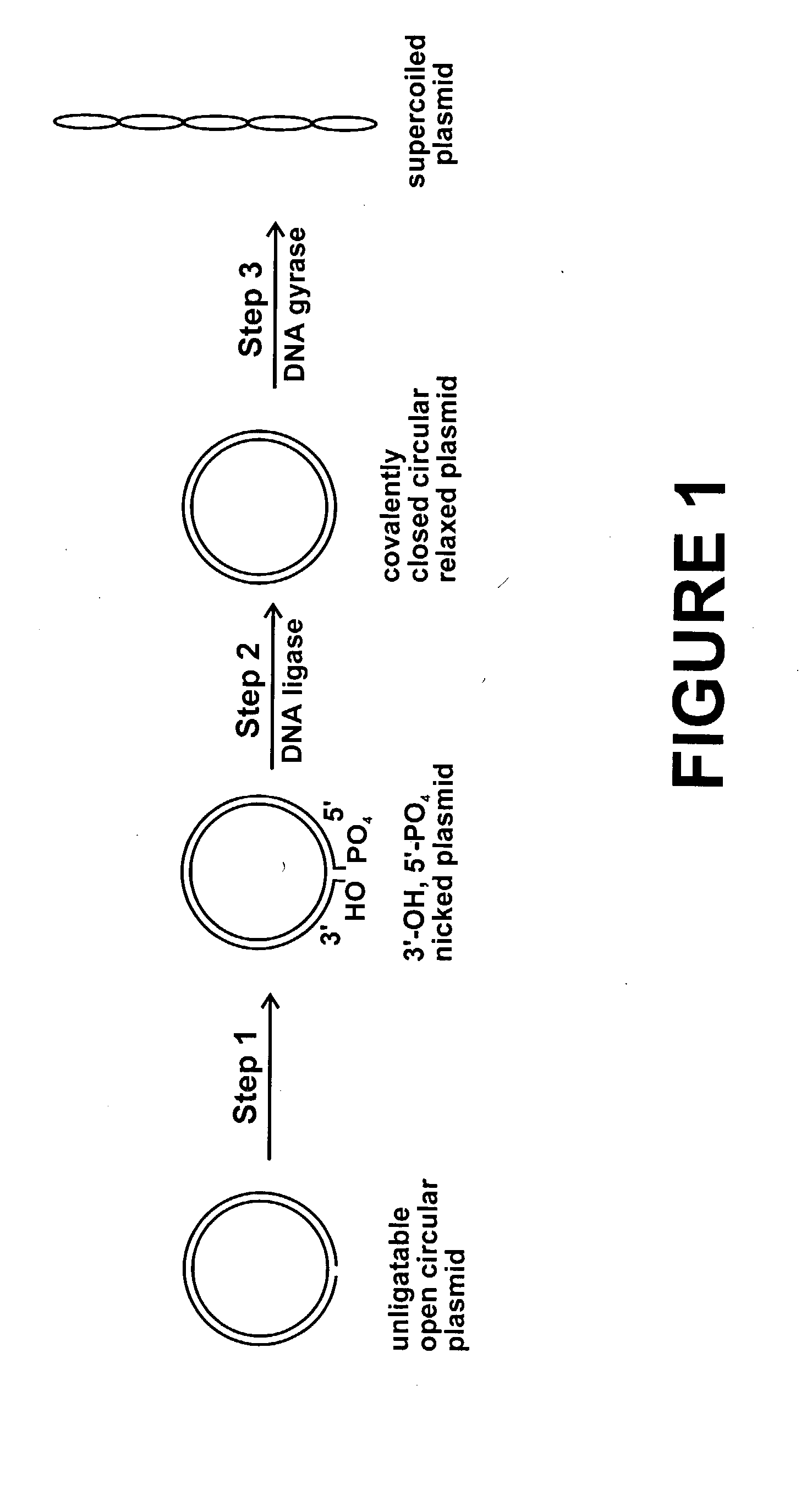
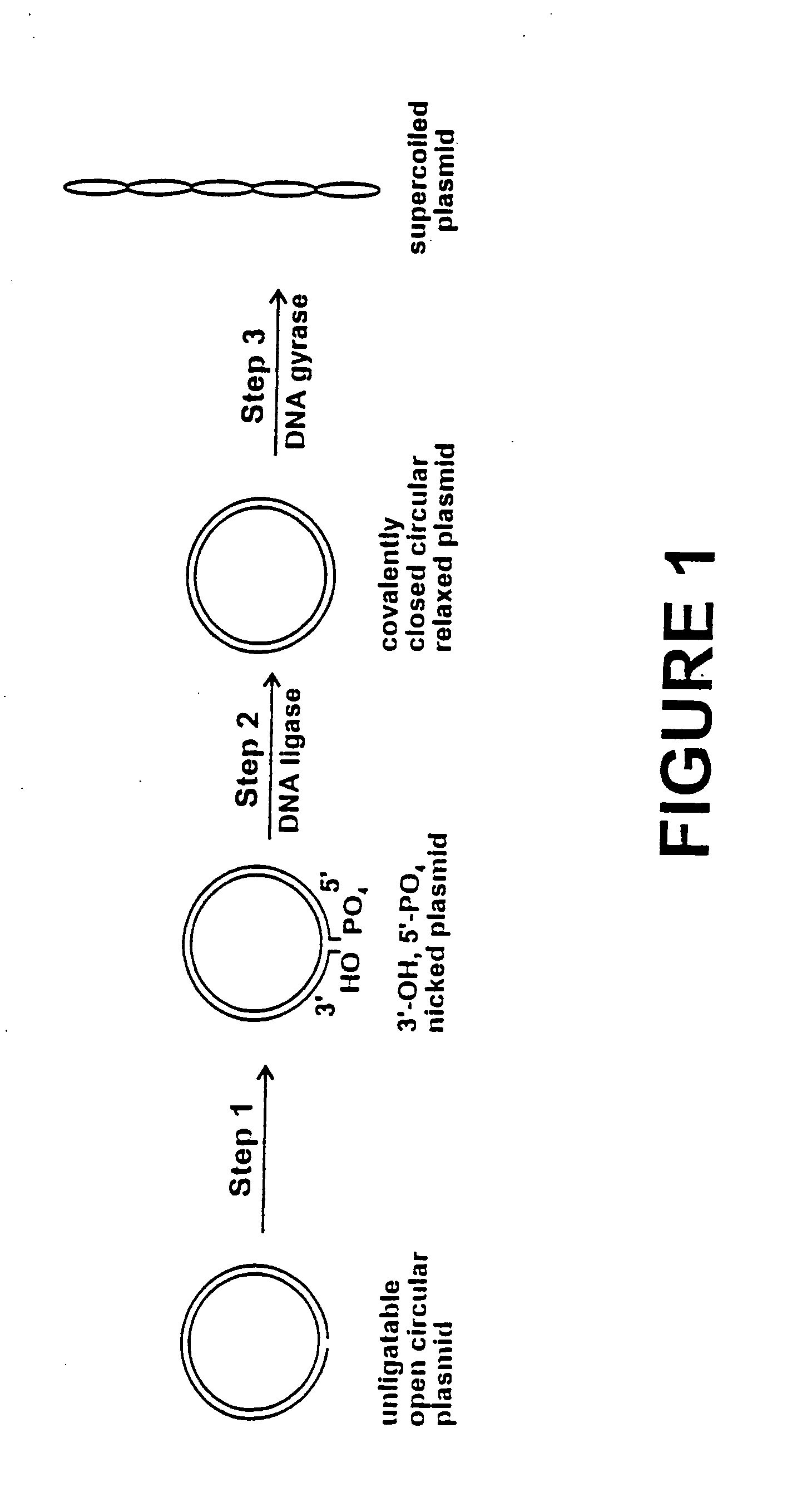
Method for plasmid preparation by conversion of open circular plasmid
InactiveUS20040191871A1Increase percentageFermentationVector-based foreign material introductionNucleotidePhosphoric acid
In accordance with the invention, there is provided a method for preparing plasmid from host cells which contain the plasmid, comprising the steps: (a) preparing a cleared lysate of the host cells, wherein the cleared lysate comprises unligatable open circular plasmid, wherein the open circular plasmid is not 3'-hydroxyl, 5-phosphate nicked plasmid; (b) incubating the unligatable open circular plasmid with one or more enzymes in the presence of their appropriate nucleotide cofactors, whereby the unligatable open circular plasmid is converted to 3'-hydroxyl, 5'-phosphate nicked plasmid; (c) incubating the 3'-hydroxyl, 5'-phosphate nicked plasmid with DNA ligase in the presence of DNA ligase nucleotide cofactor, whereby 3'-hydroxyl, 5'-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) incubating the relaxed covalently closed circular plasmid with DNA gyrase in the presence of DNA gyrase nucleotide cofactor, whereby relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. Preferably, the enzymatic steps (b), (c), and (d) are performed in a single step using an enzyme mixture comprising DNA polymerase, DNA ligase, and DNA gyrase. Preferably, the mixture further comprises a 3' terminus deblocking enzyme, such as exonuclease III or 3'-phosphatase. Preferably, the mixture further comprises one or more regenerating enzymes and a high energy phosphate donor, whereby the nucleotide by-products of the nucleotide cofactors generated by DNA ligase and DNA gyrase are converted to back to nucleotide cofactor. Preferably, the enzyme mixture further comprises one or more exonucleases, such as ATP dependent exonuclease, whereby linear chromosomal DNA is selectively degraded.
Owner:HYMAN EDWARD DAVID
Construction method and application of targeting hepatoma oncolytic adenovirus
ActiveCN103981155AEfficient liver cancer specificityImprove targetingGenetic material ingredientsViruses/bacteriophagesEscherichia coliEnzyme digestion
The invention discloses a construction method of targeting hepatoma oncolytic adenovirus, which comprises the following steps: 1), pXC2-GP73 plasmid carrying GP73 core promoter is prepared; 2), pSD55-GP73-gene plasmid is prepared; 3), the pSD55-GP73-gene plasmid is linearized by PmeI and transformed into plasmid Adeasy-1 escherichia coli BJ5183 containing whole sequence adenovirus backbone DNA for recombination to produce an adenovirus backbone DNA plasmid vector, wherein E1A region of the adenovirus backbone DNA plasmid vector is controlled by the GP73 core promoter, and target genes carried by E1B55 and E3 regions of the adenovirus backbone DNA plasmid vector are deleted; 4), the recombinant plasmid, which is identified to be correct, is linearized by enzyme digestion with Pac I, and then transfected into 293A cells for virus packaging to obtain the targeting oncolytic adenovirus GD55-gene when the cell lesion is appeared. The invention also provides application of the oncolytic adenovirus GD55-gene in preparation drugs for curing liver cancer.
Owner:ZHEJIANG SCI-TECH UNIV
Method for plasmid preparation by conversion of open circular plasmid to supercoiled plasmid
InactiveUS20050084938A1Increase productionUniversal procedureHydrolasesMicrobiological testing/measurementPhosphateGenetics
In one embodiment of the invention, a method is provided for preparing plasmid from host cells which contain the plasmid, comprising: (a) providing a plasmid solution comprised of unligatable open circular plasmid; (b) reacting the unligatable open circular plasmid with one or more enzymes and appropriate nucleotide cofactors, such that unligatable open circular plasmid is converted to 3′-hydroxyl, 5′-phosphate nicked plasmid; (c) reacting the 3′-hydroxyl, 5′-phosphate nicked plasmid with a DNA ligase and DNA ligase nucleotide cofactor, such that 3′-hydroxyl, 5′-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) reacting the relaxed covalently closed circular plasmid with a DNA gyrase and DNA gyrase nucleotide cofactor, such that relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. In other embodiments, DNA gyrase is replaced by reverse DNA gyrase or reaction (d) is not performed.
Owner:HYMAN EDWARD D
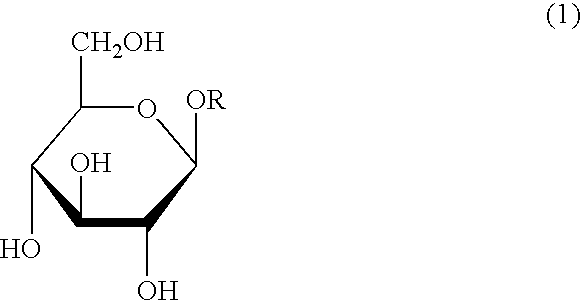
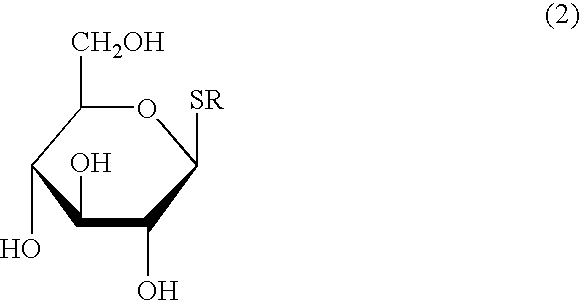
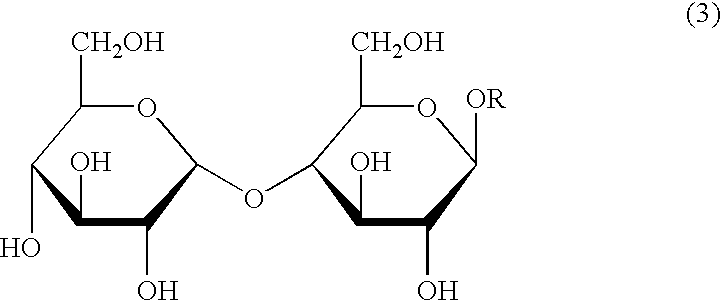
Process for the reduction of endotoxins in a plasmid preparation using a carbohydrate non-ionic detergent with silica chromatography
The present invention provides methods for the reduction of endotoxins in a plasmid preparation using a carbohydrate non-ionic detergent with silica chromatography.
Owner:SIGMA ALDRICH CO LLC
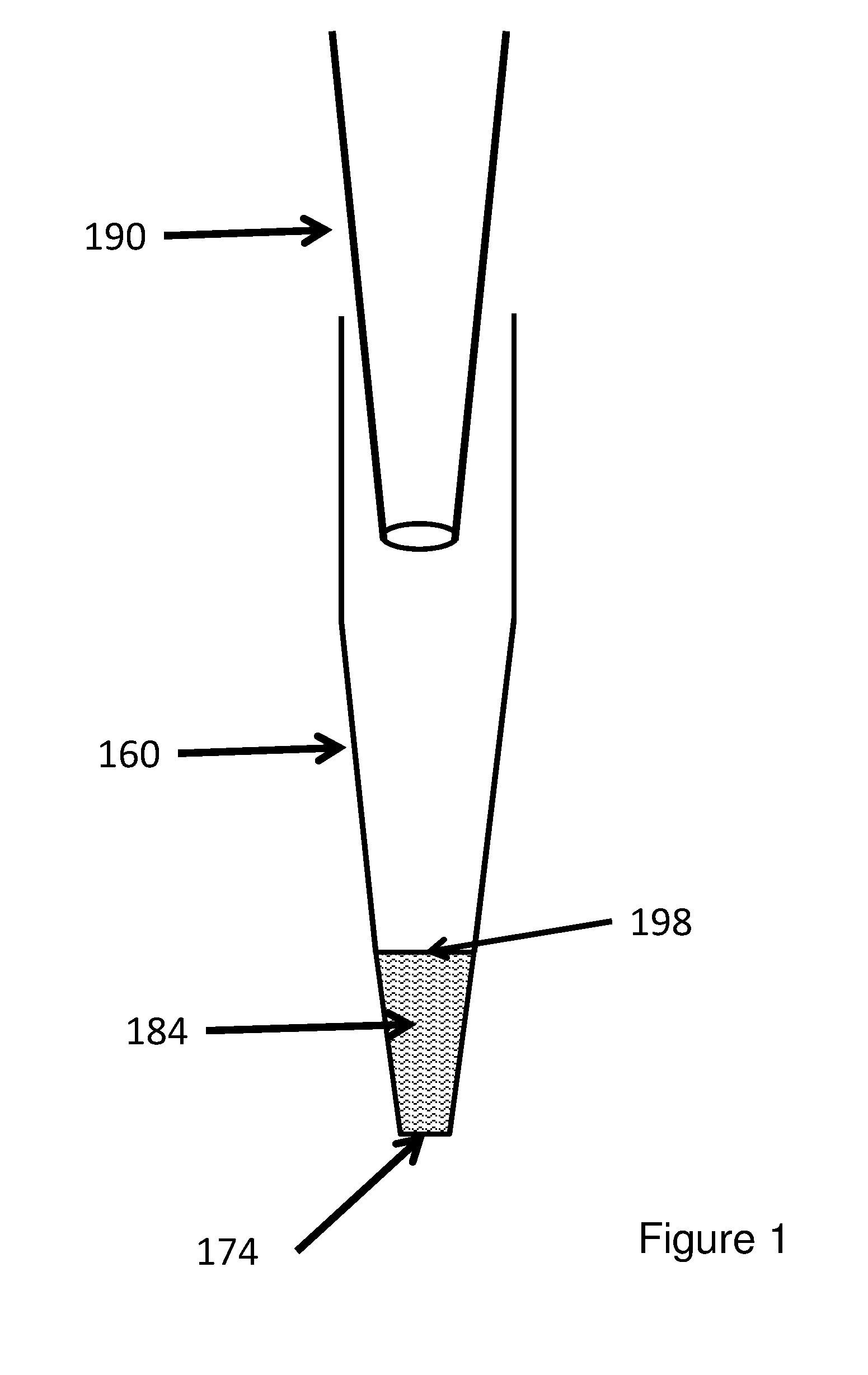
Devices and methods for plasmid purification
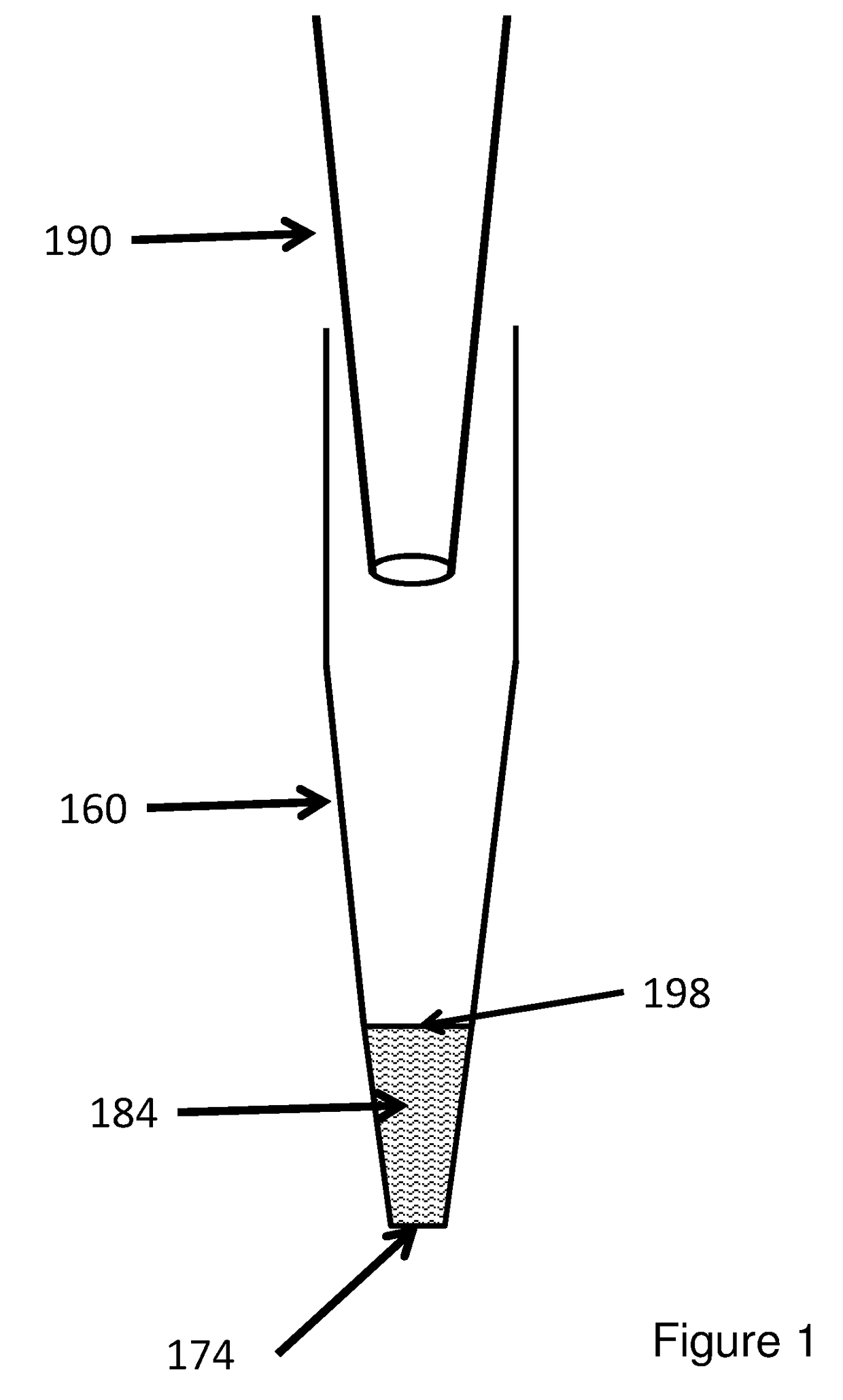
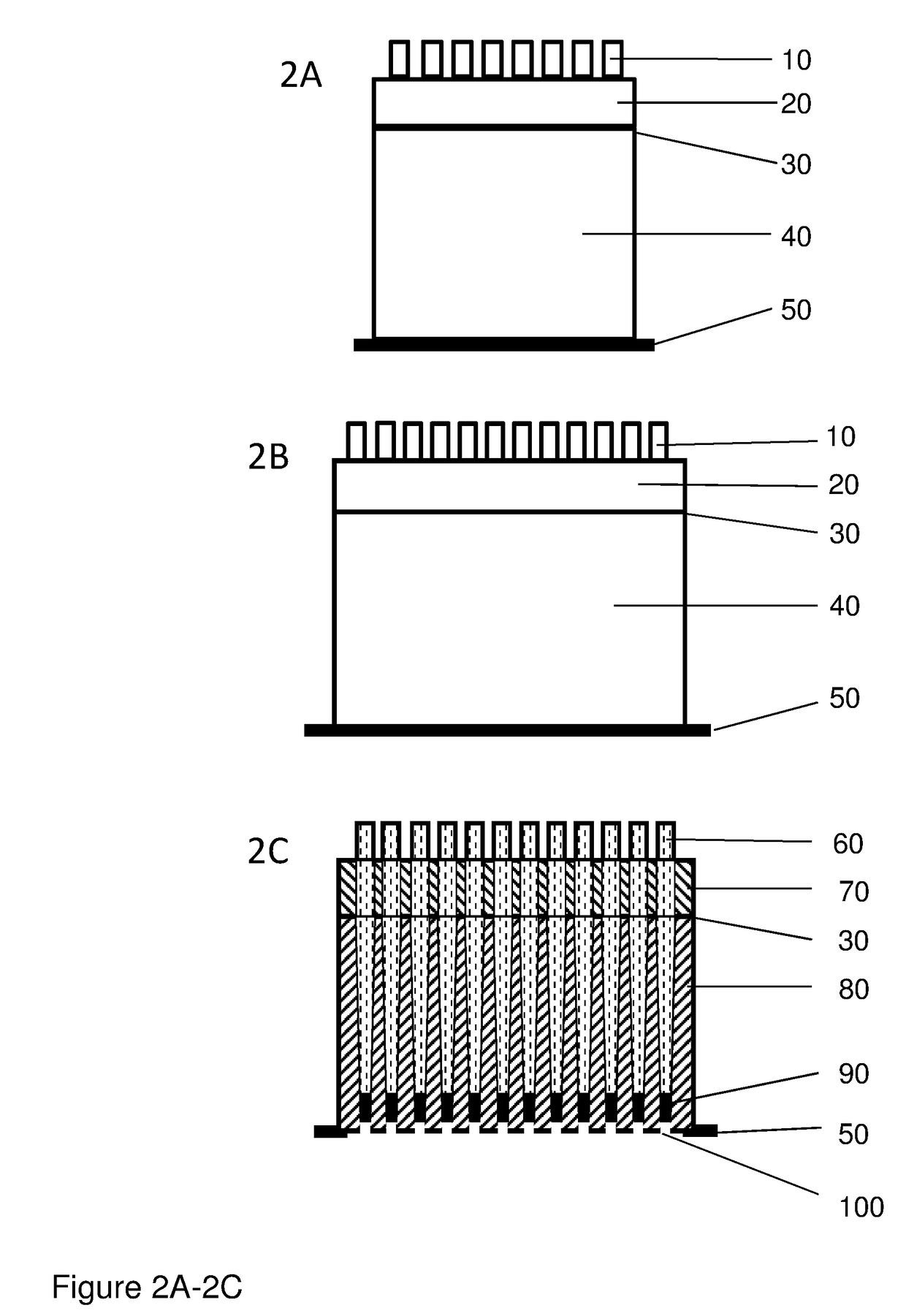
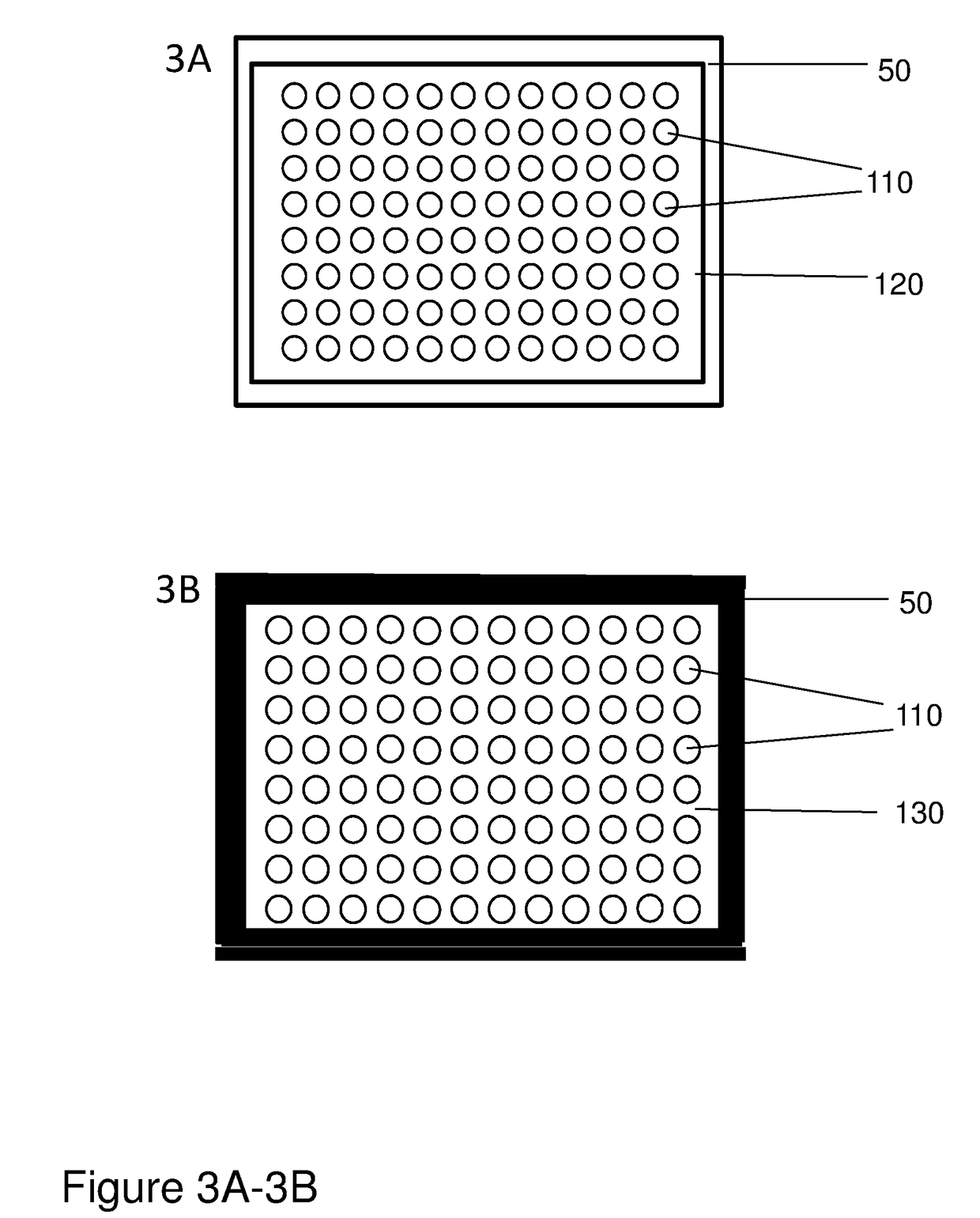
The invention provides columns (including pipette tip columns) and automated methods for the purification of nucleic acids including plasmids. Nucleic acids can be purified from unclarified, clarified or partially-clarified cell lysates that contain cell debris. The columns typically include a bed of medium positioned above a bottom frit and with an optional top frit. Plasmid preparation scales include miniprep, midiprep, maxiprep, megaprep and gigaprep.
Owner:PHYNEXUS
Reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies
The invention relates to a reagent kit for detecting antiuninuclear cell proliferation Listeria bacteria by colloidal gold Hly gene monoclonal antibodies and belongs to the technical field of hygiene inspection and medical inspection. The reagent kit is characterized by comprising the steps of: 1, primer design; 2, polymerase chain reaction (PCR) amplification; 3, agarose gel electrophoresis reaction conditions; 4, target deoxyribonucleic acid (DNA) segment cutting; 5, heat shock conversion method application; 6, reagent kit purification and plasmid preparation by a sodium dodecyl sulfate (SDS) cracking method; 7, protein induction expression; 8, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE); 9, required strip cutting from the SDS-PAGE; and 10, colloidal gold preparation. The regent kit has the beneficial effect that the limitation aiming at the single-clostridium and single-antigen test in the prior art is avoided. The antibody is pure, the valence is high, and the sensitivity and the accurate degree are improved. The single added Listeria bacterium is detected. Compared with an enzyme-linked immuno sorbent assay (ELISA) method and a PCR method, the reagent kit has the advantage that the Listeria bacterium detection is faster and more convenient by the immune colloidal gold chromatography.
Owner:何鑫 +4
Method for plasmid preparation by conversion of open circular plasmid to supercoiled plasmid
InactiveUS20060057683A1Increase productionUniversal procedureFermentationVector-based foreign material introductionPhosphateGenetics
In one embodiment of the invention, a method is provided for preparing plasmid from host cells which contain the plasmid, comprising: (a) providing a plasmid solution comprised of unligatable open circular plasmid; (b) reacting the unligatable open circular plasmid with one or more enzymes and appropriate nucleotide cofactor(s), such that unligatable open circular plasmid is converted to 3′-hydroxyl, 5′-phosphate nicked plasmid; (c) reacting the 3′-hydroxyl, 5′-phosphate nicked plasmid with a DNA ligase and DNA ligase nucleotide cofactor, such that 3′-hydroxyl, 5′-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) reacting the relaxed covalently closed circular plasmid with a DNA gyrase and DNA gyrase nucleotide cofactor, such that relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. In other embodiments, DNA gyrase is replaced with reverse DNA gyrase or reaction (d) is not performed.
Owner:HYMAN EDWARD D
Engineering bacterium capable of co-producing long-chain dicarboxylic acid and 1,3-propylene glycol and building method thereof
ActiveCN106636156AIncrease productionImprove use valueMicroorganism based processesNucleic acid vectorDicarboxylic acidBiology
The invention relates to an engineering bacterium capable of co-producing long-chain dicarboxylic acid and 1,3-propylene glycol and a building method thereof. The engineering bacterium is prepared by transforming a recombinant plasmid. The recombinant plasmid is prepared by the following steps: performing PCR (Polymerase Chain Reaction) amplification to obtain a glycerol dehydratase gene, and connecting the glycerol dehydratase gene with a promoter pGAPg obtained by PCR amplification in an overlapping way to obtain a pGAPg-GD segment; performing PCR amplification to obtain a 1,3-propanediol dehydrogenase gene, connecting the 1,3-propanediol dehydrogenase gene with the promoter pGAPp obtained by the PCR amplification in the overlapping way to obtain a pGAPp-PDE segment; performing PCR amplification to obtain a terminator gene, and preparing a two-way terminator segment; separately connecting the segments to obtain the recombinant plasmid. By adopting the engineering bacterium built by the method, co-expression of a plurality of genes is realized; moreover, the yield of the long-chain dicarboxylic acid is 10g / L, and the yield of the 1,3-propylene glycol generated by transforming glycerine is 13g / L, being remarkably higher than current reported values.
Owner:QILU UNIV OF TECH
Process for the reduction of endotoxins in a plasmid preparation using a carbohydrate non-ionic detergent with silica chromatography
ActiveUS20050245733A1Decrease endotoxin levelThe process is simple and fastSugar derivativesMicrobiological testing/measurementNon ionicSilicon dioxide
The present invention provides methods for the reduction of endotoxins in a plasmid preparation using a carbohydrate non-ionic detergent with silica chromatography.
Owner:SIGMA ALDRICH CO LLC
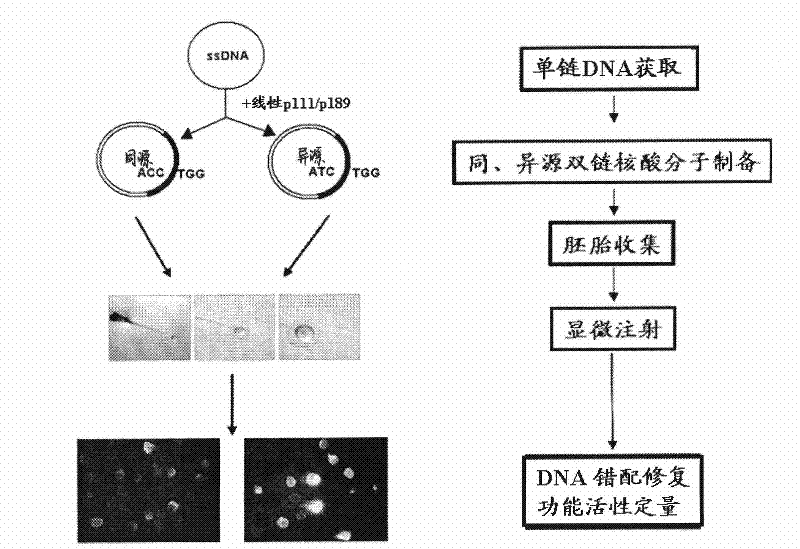
Analysis method for activity of mismatch repair of zebra fish embryo DNA and application thereof
InactiveCN102392073AReduce volumeEasy to feedMicrobiological testing/measurementBiotechnologyHeterologous
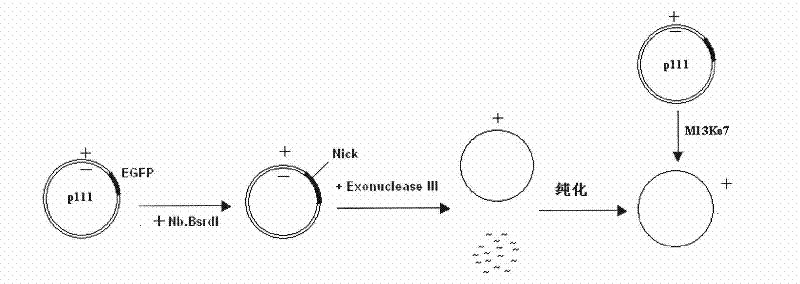
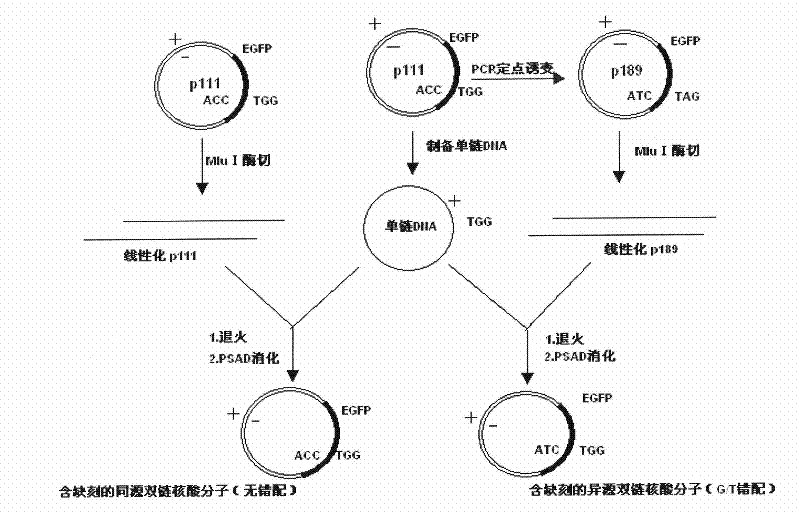
The present invention relates to an analysis method for the activity of mismatch repair of zebra fish embryo DNA, and the application of the method in toxicity evaluation of mismatch repair of environmental compound DNA. The method includes the following steps: preparing single-stranded DNA molecules with plasmids pGEM-EGFP; preparing homoduplex and heteroduplex with the single-stranded DNA molecules and linearized plasmids pGEM-EGFP and plasmids pGEM- (mt) EGFP respectively; microinjecting the homoduplex and heteroduplex to a zebra fish embryo with 1-cell stage; and quantifying the activity of mismatch repair of embryo DNA. The present invention establishes the analysis method for the activity of mismatch repair of DNA on the zebra fish embryo level for the first time, and successfully applies the method to the toxicity evaluation of mismatch repair of environmental compound DNA, which is significant for the evaluation of health risk and cancerization risk for environmental compound people. In addition, by applying the zebra fish to the evaluation of the toxicity of mismatch repair of compound DNA, the method has the advantages of simple operation, direct viewing, fastness, simple administration for tested compounds, small dosage, and the like.
Owner:WENZHOU MEDICAL UNIV
Construction method and application of oenococcus oeni engineering bacteria
ActiveCN110846331AReduce storage timeShorten the production cycleBacteriaHydrolasesBiotechnologyEnzyme Gene
The invention relates to a construction method and application of oenococcus oeni engineering bacteria. The construction method comprises the following steps: (1) preparing lipase gene lipase; (2) preparing homologous arm genes with lipase genes; (3) preparing plasmid pMG36e-lipase; (4) preparing nisl fragments; (5) preparing a linear fragment without containing an erythromycin sequence; (6) preparing an expression vector pMG36n-lipase; and (7) transforming the expression vector pMG36n-lipase into oenococcus oeni competent cells to obtain the oenococcus oeni engineering bacteria. The oenococcus oeni engineering bacteria with a function of degrading esters obtained by the invention can be applied to production of wine, so that adverse effects on taste, aroma and the like of the wine causedby excessive esters in the current wine brewing process are avoided, the preservation time of the wine is shortened, and the production period of the wine reaching the same taste is shortened.
Owner:QILU UNIV OF TECH
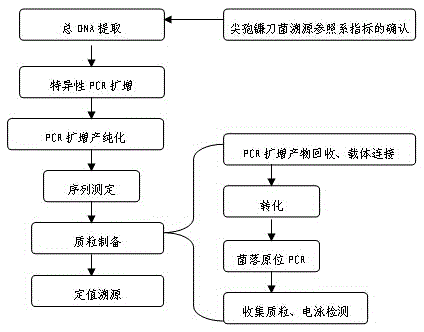
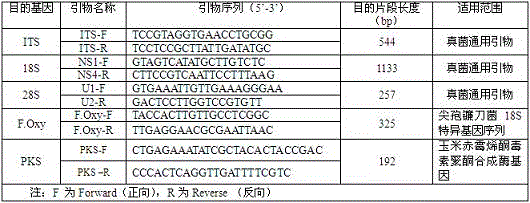
Fusarium oxysporum nucleotide sequence qualitative standard sample and preparing method thereof
InactiveCN105567849ANo longer biologically activeBiologically activeMicrobiological testing/measurementVector-based foreign material introductionPositive controlSource tracing
The invention belongs to the field of biotechnology, and particularly relates to a fusarium oxysporum nucleotide sequence qualitative standard sample and a preparing method thereof. Fusarium oxysporum in the logarithmic phase is acquired, total DNA extraction is conducted, specific PCR amplification sequencing is conducted, and then plasmid preparation is conducted. The prepared nucleotide sequence standard sample does not have biological activity any more, biological pollution and infection are avoided, the source of molecular biology positive control is available, and the sample is stable, free of pollution and easy to store. Based on the fact that fusarium oxysporum has the nucleotide sequence technical index with taxonomical significance, the problems of fusarium oxysporum nucleotide sequence standard sample source tracing reference system and reference index are solved, the fusarium oxysporum nucleotide sequence standard sample with real source tracing significance is obtained through application in setting value analysis of the standard sample, and the fusarium oxysporum nucleotide sequence qualitative standard sample has great significance in development of nucleotide sequence classified gradation standard samples in China.
Owner:蒋丹 +3
Modulation of Androgen Receptor for Treatment of Prostate Cancer
The present invention provides methods for the reduction of endotoxins in a plasmid preparation using a carbohydrate non-ionic detergent with silica chromatography.
Owner:THE CLEVELAND CLINIC FOUND +1
IL-24 and OSM double-gene co-expression recombinant plasmid, recombinant adenovirus and application
The invention relates to the field of biology, and discloses a double-gene co-expression recombinant plasmid pAdTrack-CMV-IL-24-polyA-promoter-OSM with the preservation number as China center for type culture connection number (CCTTCC NO.): M2011378, and a recombinant adenovirus Ad-IL-24-polyA-promoter-OSM prepared by the plasmid. An IL-24 gene and an OSM gene are subcloned between Ad-polyA-promoter empty carriers to construct the IL-24 and OSM double-gene co-expression recombinant plasmid, homologous recombination is performed between the recombinant plasmid and an adenovirus pAdEasy-1 to generate the recombinant adenovirus in packaging mode in a QBI-293A cell. The recombinant adenovirus has synergistic effect on inhibition of melanoma and cancer cells of nasopharyngeal darcinoma, and simultaneously has synergistic effect on radiosensitivity of the cancer cells of the nasopharyngeal darcinoma.
Owner:SUZHOU UNIV
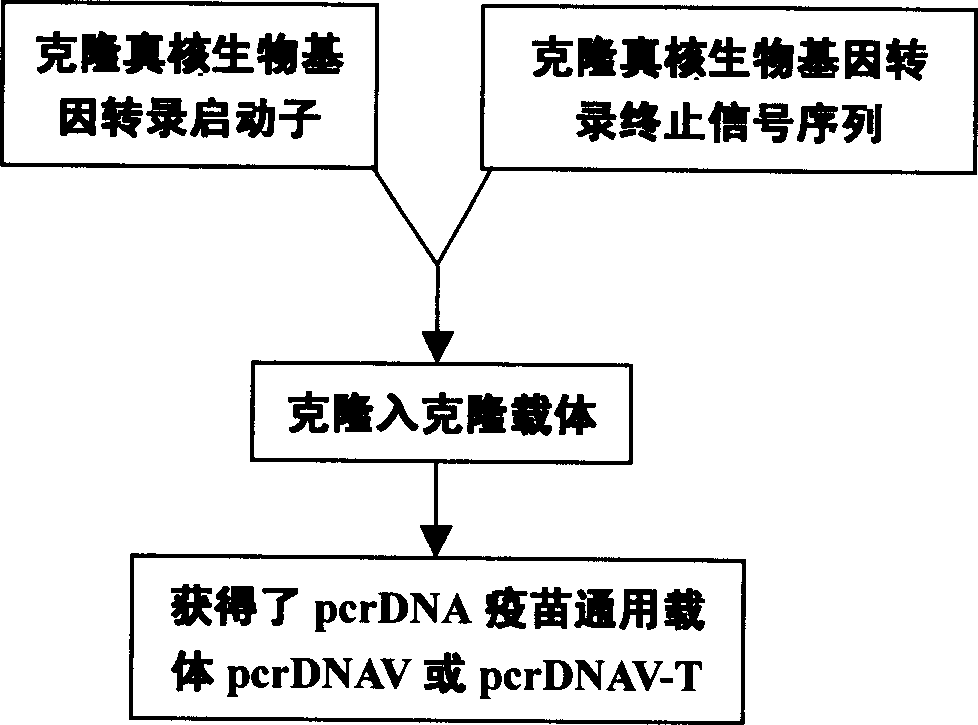
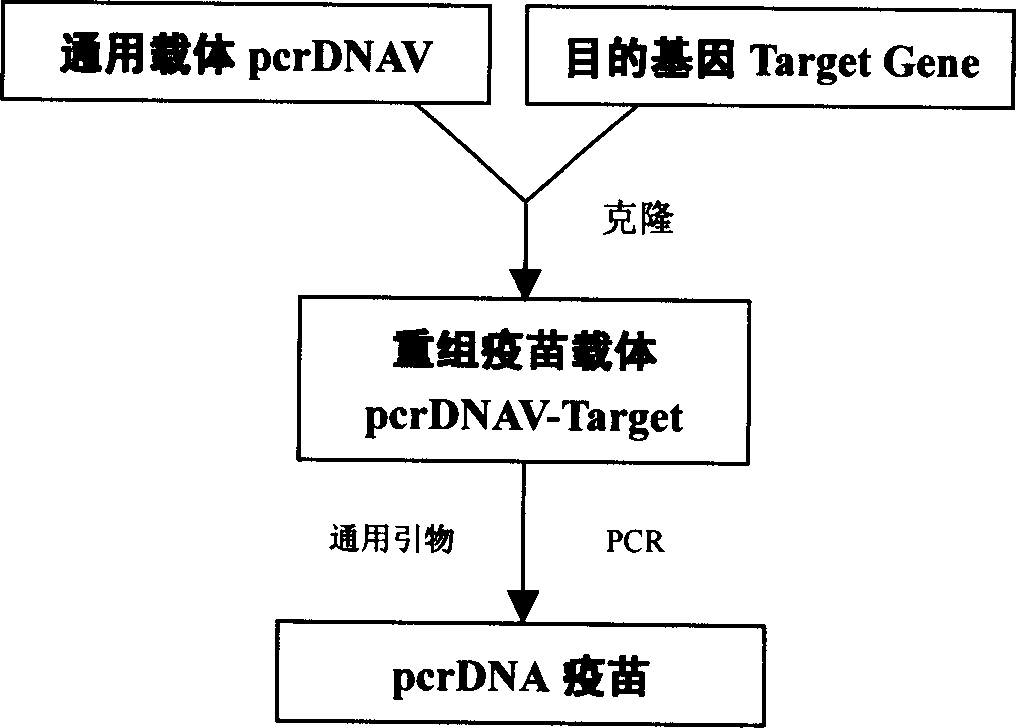
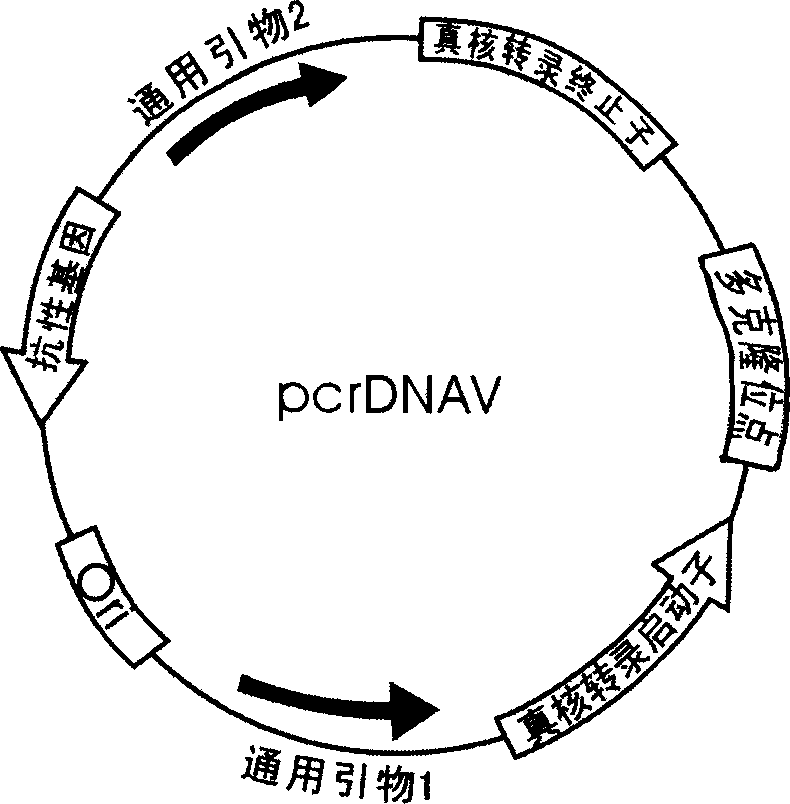
PcrDNA vaccine, and its preparing method and use
InactiveCN1850279AEasy to makeSpeed up preparationGenetic material ingredientsAntineoplastic agentsBiologyNucleic acid sequence
The present invention discloses a new type DNA vaccine-pcr DNA vaccine, its preparation method and application. The pcr DNA vaccine directly uses the PCR product in which the complete gene expression element is carried as DNA vaccine. Said DNA vaccine does not carry any indifferent gene or nucleic acid sequence except for target gene, so that it has better biological safety. Said invention adopts PCR technique to prepare DNA vaccine. The pcr DNA vaccine can be used for immunizing human body and animal, and can be used for preventing and curing human and diseases, such as various infectious diseases, parasitic diseases, tumor, nutritional metabolic disease and gene defect disease.
Owner:广东省农业科学院兽医研究所
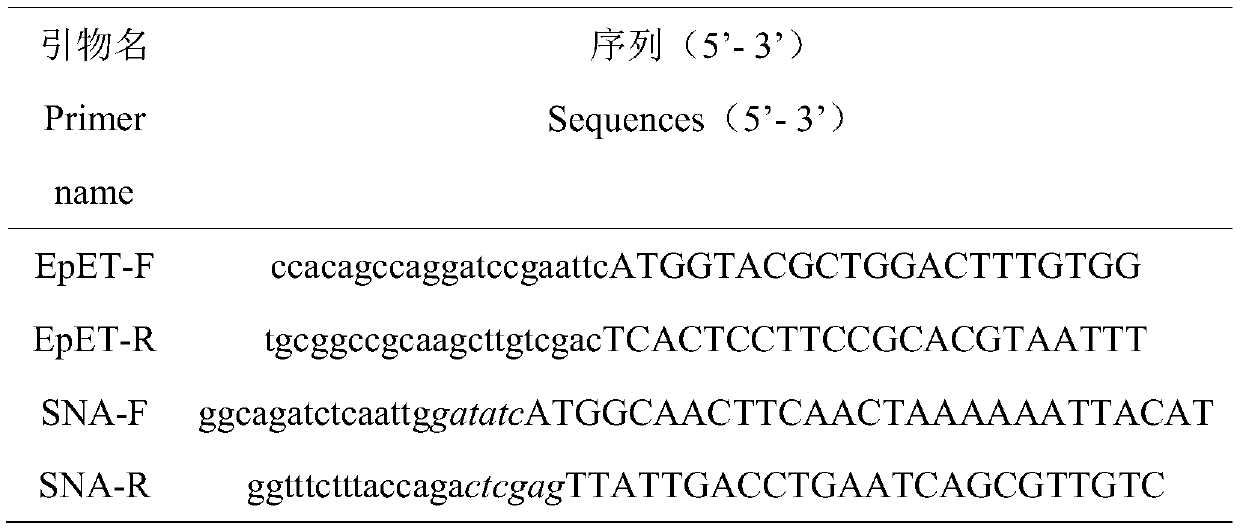
Escherichia coli ghost capable of realizing dual-protein expression and preparation method of escherichia coli ghost capable of realizing dual-protein expression
InactiveCN110951666AImprove securityAchieve degradationBacteriaHydrolasesProtein structureStaphyloccocus aureus
The invention discloses an escherichia coli ghost capable of realizing dual-protein expression. The ghost is prepared from dual-expression bacteriolysis plasmids, the dual-expression bacteriolysis plasmids use pETDuet1 as a carrier, besides, an E gene and staphylococcus aureus nuclease A are inserted, and bacteriolysis plasmids capable of realizing dual-protein expression of the E gene and the staphylococcus aureus nuclease A are realized. According to the ghost disclosed by the invention, gene fragments are respectively inserted in two independent multiple clone sites of the carrier, so thatthe problem that two protein structures in expression mutually influence can be effectively solved, besides, degradation of bacterium DNA is realized, the obtained ghost products are high in safety, dual-protein expression is realized, and the dual-protein expression efficiency is high. The escherichia coli ghost prepared from the pETDuet1-E-SNA bacteriolysis plasmids disclosed by the invention can degrade heredity substances (including drug resistance genes and toxin genes) of the escherichia coli, the use safety of the ghost can be effectively increased, and biological potential safety hazard existing in use of the ghost products can be avoided.
Owner:JIANGSU AGRI ANIMAL HUSBANDRY VOCATIONAL COLLEGE
Process for preparing plasmid in large scale with high yield
InactiveCN1373214AFairly pureIncrease productionBacteriaSugar derivativesEscherichia coliProteinase K
Owner:MILITARY VETERINARY INST MILITARY SUPPIES PLA
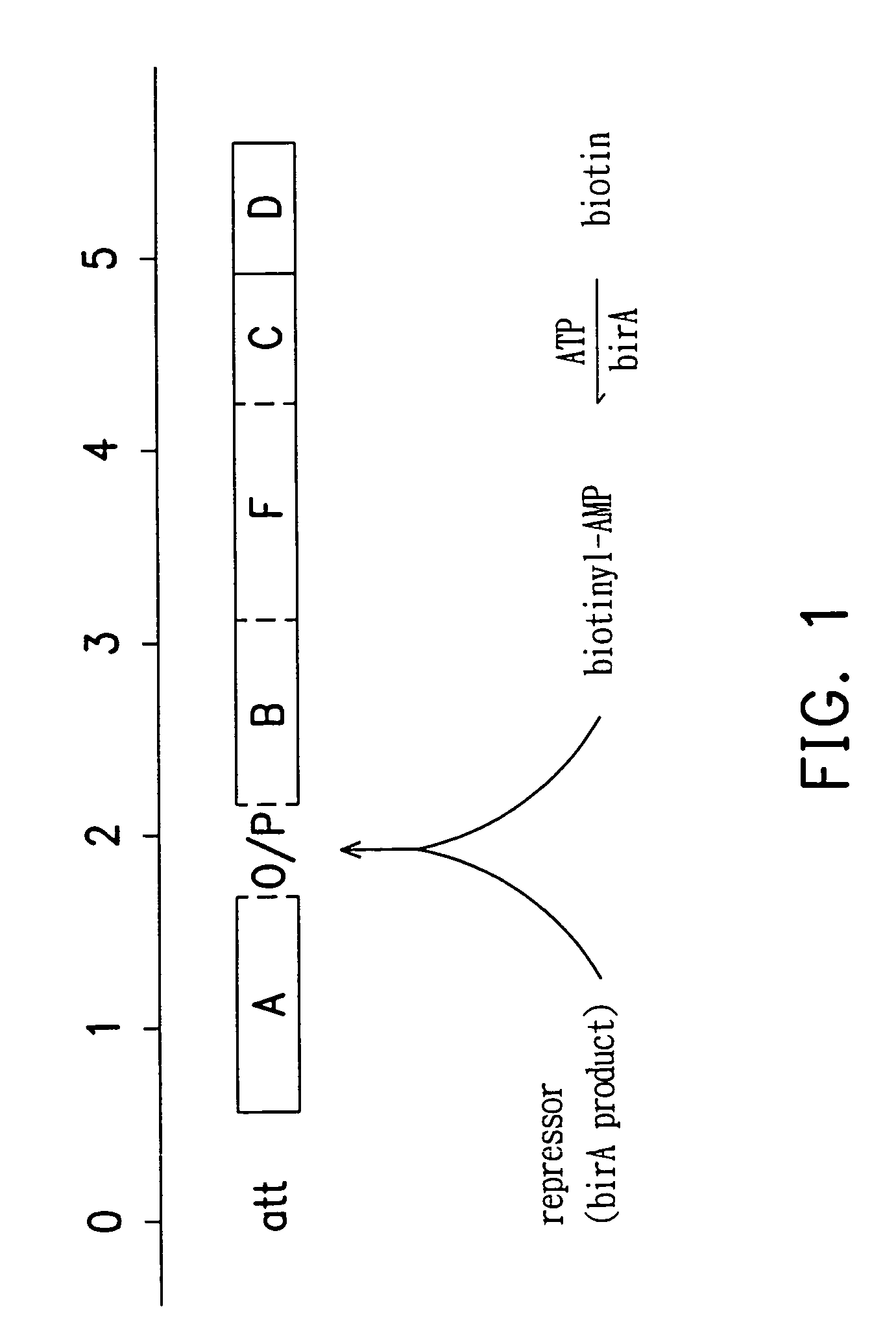
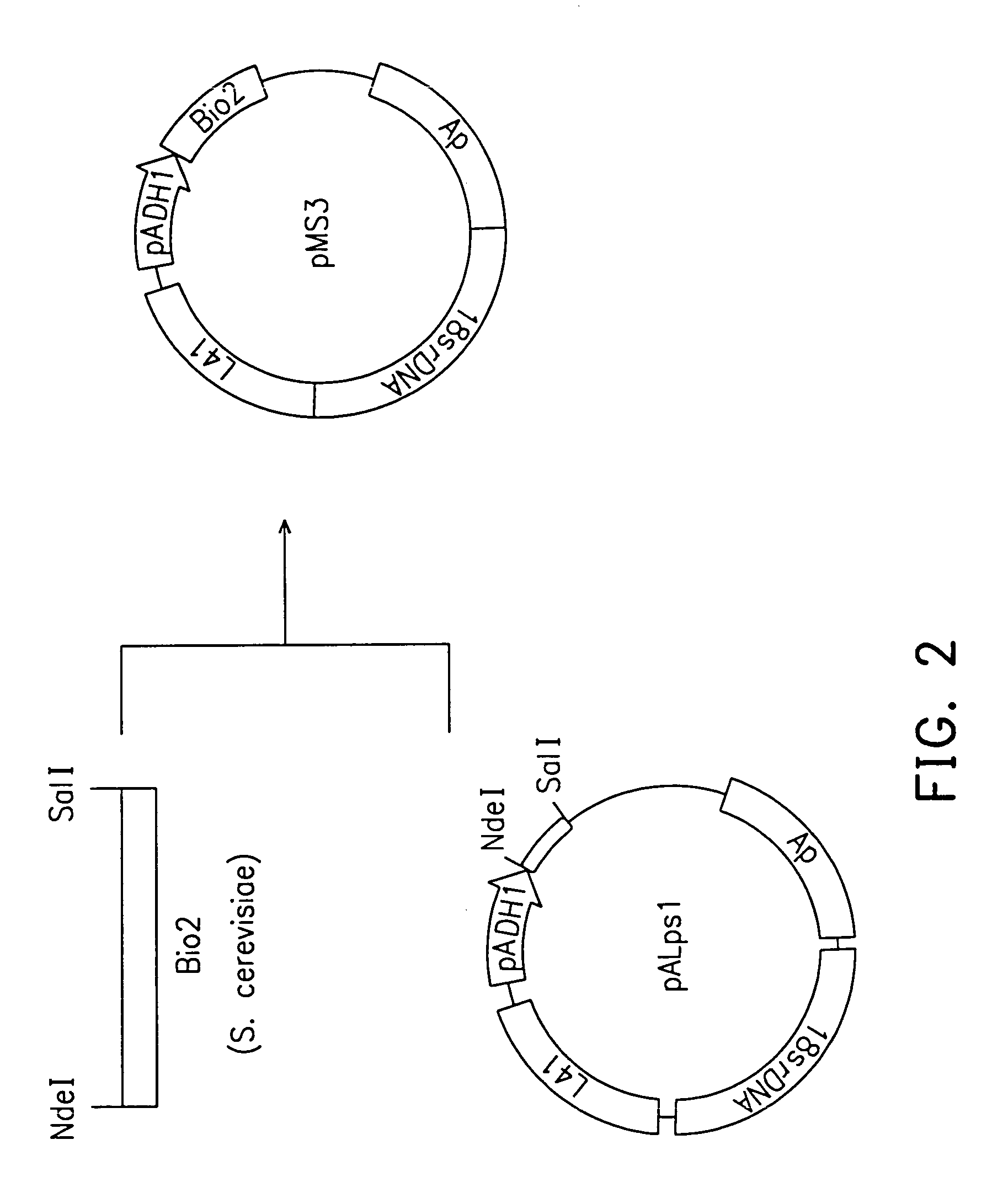
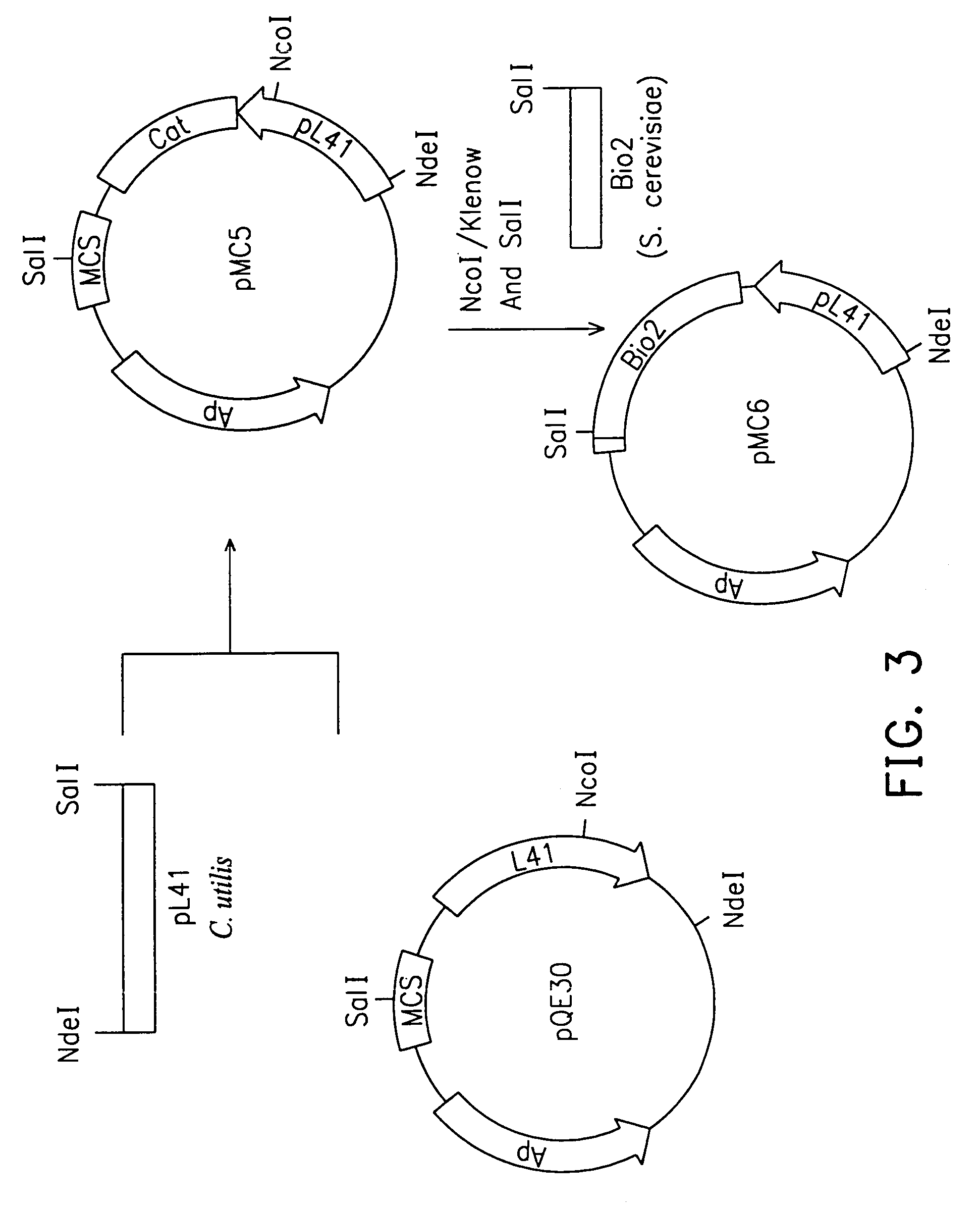
Methods for preparing yeast with improved biotin productivity using integrating plasmids encoding biotin synthase
Owner:NAT SCI COUNCIL
Methods and devices for nucleic acid purification
The invention provides pipette tip columns and automated methods for the purification of nucleic acids including plasmids. Nucleic acids can be purified from unclarified, clarified or partially-clarified cell lysates that contain cell debris. The columns typically include a bed of medium positioned in the pipette tip column, above a bottom frit and with an optional top frit. Plasmid preparation scales include miniprep, midiprep, maxiprep, megaprep and gigaprep.
Owner:PHYNEXUS
Preparation and detection method of pseudomonas aeruginosa algC gene clonal strains
InactiveCN104372018AMicrobiological testing/measurementMicroorganism based processesPseudomona aeruginosaBacterial strain
The invention discloses a preparation method of clonal strains containing pseudomonas aeruginosa algC gene. The method comprises the following steps: culturing bacterial strain DNA, preparing chromosome, preparing plasmid DNA, optimizing PCR, purifying PCR products, cloning algC gene and preserving clonal strains. The invention also discloses a detection method of clonal strains containing pseudomonas aeruginosa algC gene. The invention provides materials and technique for the research and development of pseudomonas aeruginosa alginate structural gene and alginate products and provides experimental materials, techniques and molecular biological basis theory for environmental safety evaluation test.
Owner:GUIZHOU INST OF ANIMAL HUSBANDRY & VETERINARY
Quantitative detection method for sulfonamide-antibiotic-resistant genes in seawater bathing spot
PendingCN111304343AHigh amplification efficiencyImprove measurement accuracyMicrobiological testing/measurementSequence analysisAntibiotic resistance genes
The invention discloses a quantitative detection method for sulfonamide-antibiotic-resistant genes in a seawater bathing spot. The method comprises the steps of subjecting a water sample and depositsof the seawater bathing spot to collecting and low-temperature storage, carrying out DNA extraction and purification on the collected sample, then, amplifying DNA fragments of the antibiotic-resistantgenes, cloning the DNA fragments to a host cell to construct standard plasmids, preparing a fluorescent quantitative PCR standard curve of a target DNA standard substance, and calculating the contentof the antibiotic-resistant genes in the sample according to the PCR standard curve of the standard substance and a CT value of the purified sample. According to the method disclosed by the invention, the steps are simple, the operation is convenient, a sample DNA sequence analyzing step is omitted, and the content of the antibiotic-resistant genes in the sample water and the deposits is obtainedaccording to the PCR standard curve and the CT value of the sample, so that the method is wide in applicable range.
Owner:NATIONAL MARINE ENVIRONMENTAL MONITORING CENTRE
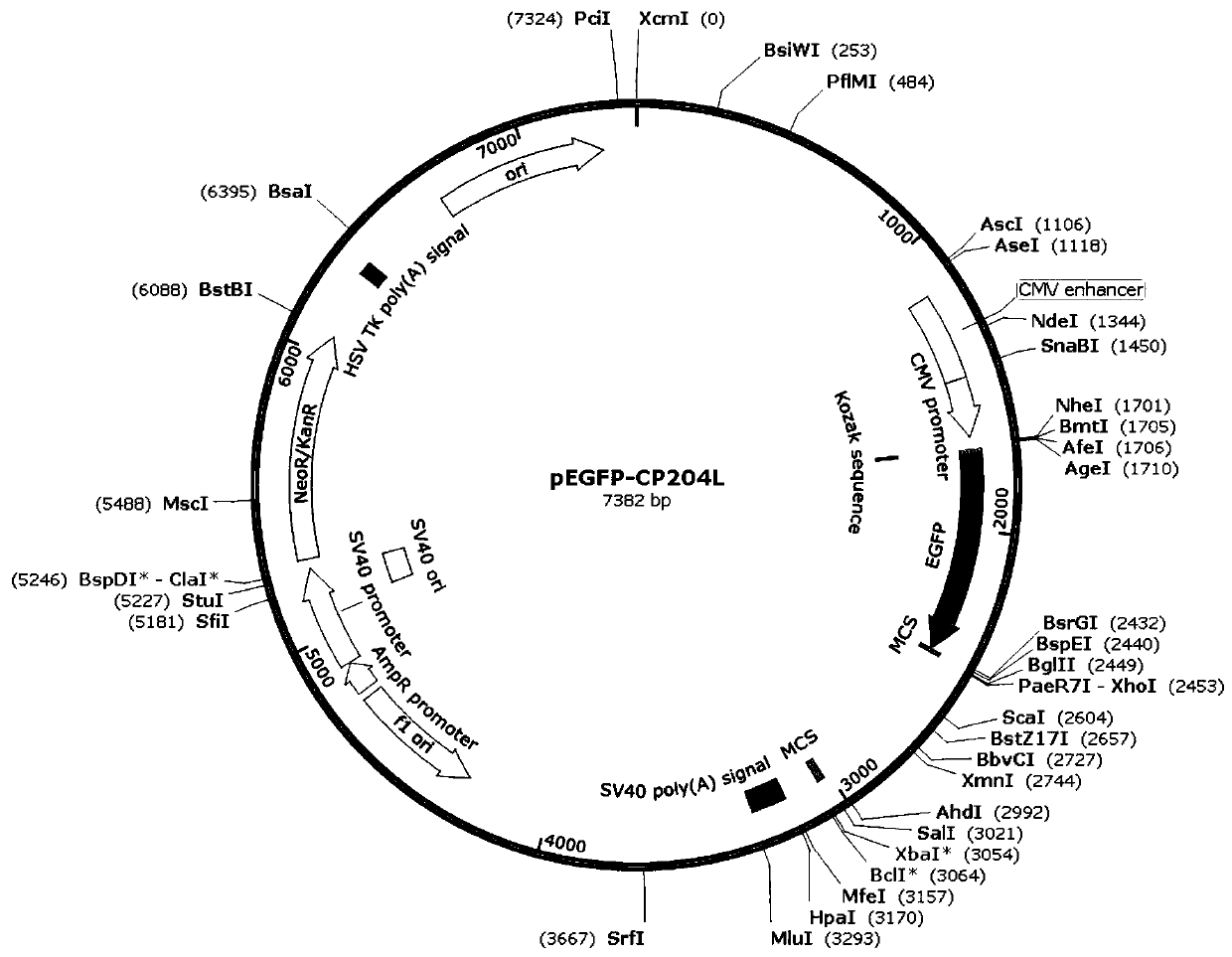
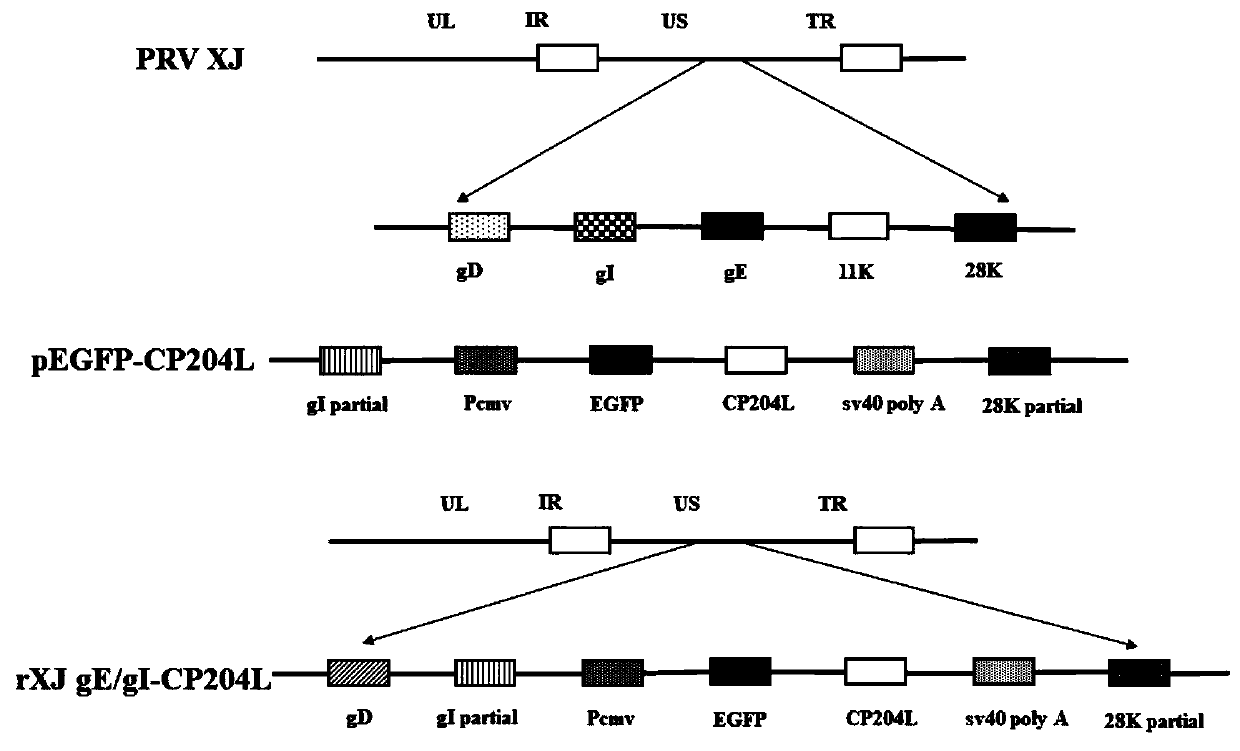
Construction method of PRV gE/gI dual-gene deletion strains for expressing ASFV P30 protein
InactiveCN111471657AAvoid infectionEasy to makeVirus peptidesAntiviralsRabies virus strainVirus strain
The invention discloses a construction method of PRV gE / gI dual-gene deletion strains for expressing ASFV P30 protein and belongs to the biopharmaceutical field. The construction method specially comprises the following steps of S1, preparing a reagent; S2, preparing cloned plasmids; S3, constructing a PRV eukaryon transfer plasmid pEGFP-CP204L; S4, performing cotransfection on DNA of PRV and pEGFP-CP204L plasmids. P30 protein gene (CP204L) fragments are synthesized, the transfer plasmid pEGFP-CP204L is constructed, a classical homologous recombination manner is adopted for inserting an ASFV CP204L gene into a PRV genome to replace gI and gE virulence genes, and recombinant pseudorabies virus strains rXJgE / gI-CP204L for constructing co-expression P30 protein can be used for preventing ASFVand PRV infection, and the cloned plasmids are simple to prepare, low in cost and suitable for industrial production.
Owner:SICHUAN AGRI UNIV
Large-scale plasmid preparation process
The invention discloses a large-scale plasmid preparation process which comprises the following steps: preparing production seeds, performing high-density fermentation, thalli harvesting, removing RNA and extracting and purifying the plasmids. The large-scale plasmid preparation process disclosed by the invention is simple in method and convenient to operate, lots of plasmids can be produced, and the production cost is reduced.
Owner:SHANDONG BINZHOU ANIMAL SCI & VETERINARY MEDICINE ACADEMY
Methods and devices for nucleic acid purification
The invention provides pipette tip columns and automated methods for the purification of nucleic acids including plasmids. Nucleic acids can be purified from unclarified, clarified or partially-clarified cell lysates that contain cell debris. The columns typically include a bed of medium positioned in the pipette tip column, above a bottom frit and with an optional top frit. Plasmid preparation scales include miniprep, midiprep, maxiprep, megaprep and gigaprep.
Owner:PHYNEXUS
Method for plasmid preparation by conversion of open circular plasmid to supercoiled plasmid
InactiveUS20050255563A1Increase productionUniversal procedureMicrobiological testing/measurementFermentationGeneticsPhosphoric acid
In one embodiment of the invention, a method is provided for preparing plasmid from host cells which contain the plasmid, comprising: (a) providing a plasmid solution comprised of unligatable open circular plasmid; (b) reacting the unligatable open circular plasmid with one or more enzymes and appropriate nucleotide cofactors, such that unligatable open circular plasmid is converted to 3′-hydroxyl, 5′-phosphate nicked plasmid; (c) reacting the 3′-hydroxyl, 5′-phosphate nicked plasmid with a DNA ligase and DNA ligase nucleotide cofactor, such that 3′-hydroxyl, 5′-phosphate nicked plasmid is converted to relaxed covalently closed circular plasmid; and (d) reacting the relaxed covalently closed circular plasmid with a DNA gyrase and DNA gyrase nucleotide cofactor, such that relaxed covalently closed circular plasmid is converted to negatively supercoiled plasmid. In other embodiments, DNA gyrase is replaced with reverse DNA gyrase or reaction (d) is not performed.
Owner:HYMAN EDWARD D
Devices and Methods for Plasmid Purification
The invention provides columns (including pipette tip columns) and automated methods for the purification of nucleic acids including plasmids. Nucleic acids can be purified from unclarified, clarified or partially-clarified cell lysates that contain cell debris. The columns typically include a bed of medium positioned above a bottom frit and with an optional top frit. Plasmid preparation scales include miniprep, midiprep, maxiprep, megaprep and gigaprep.
Owner:PHYNEXUS
Process for preparing plasmid in large scale with high yield
InactiveCN1257274CFairly pureIncrease productionBacteriaSugar derivativesEscherichia coliRestriction enzyme digestion
A high-yield large-scale plasmid preparation method of the present invention includes colony cultivation and collection: protoplast preparation: plasmid extraction and purification: DNA detector is used to measure the content of the plasmid and an appropriate enzyme is used for enzymatic identification; the process is compatible with alkali denaturation- The purity of the plasmid prepared by the CsCl density gradient ultracentrifugation method is equivalent, but the yield is greatly increased, and the plasmid yield per 1000ml of E. coli culture reaches 15-23mg. First pellet the plasmid in the supernatant using 20% PEG 8000 Instead of using isopropanol, add the precipitate to 18TE solution to dissolve, and add proteinase K with a final concentration of 50-100ug / ml to digest. Its endotoxin content is extremely low, which is equivalent to that of alkali denaturation-CsCl density gradient ultracentrifugation.
Owner:MILITARY VETERINARY INST MILITARY SUPPIES PLA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com