Preparation methods of N-(2-methylpyridyl-5-nitro-3-)-4-(3-pyridinyl)pyrimidin-2-amine and intermediate thereof
A technology of methyl iodide and compounds, which is applied in the field of preparation of pyrimidine derivatives, can solve problems such as waste, low reaction yield, and use restrictions, and achieve the effects of less pollutant discharge, simple product treatment, and reduced dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
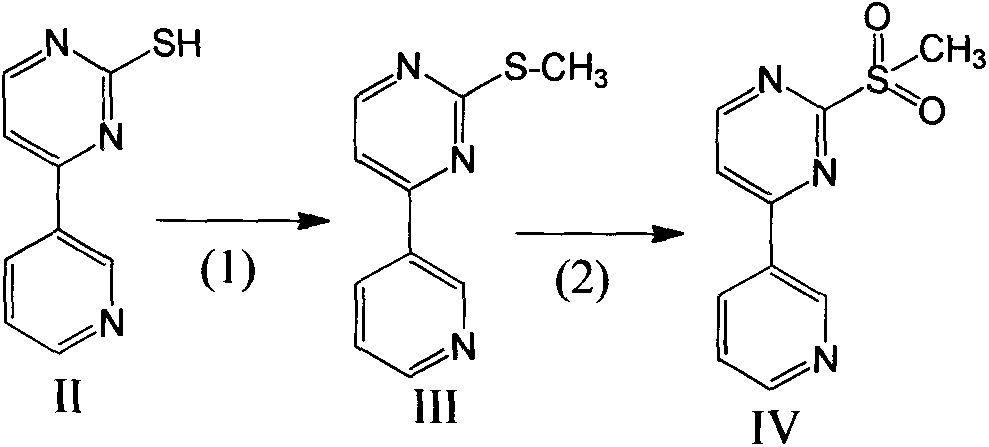
[0025] The preparation of embodiment 1-embodiment 4 compound IV, formula is as follows
[0026]
[0027] Reaction process:
[0028] Put compound II and NaOH solution as raw materials into the reaction kettle, stir to dissolve, and lower the temperature to about 0°C. Methyl iodide was added dropwise, and after the addition was completed, the reaction was kept warm until the reaction was complete. Use 0.1M hydrochloric acid to adjust the pH to 4-5, cool down to 20°C, add hydrogen peroxide, and control the addition rate so that the temperature of the reaction system does not exceed 50°C. After the addition, keep warm until the reaction is complete. Use 0.1M Na 2 CO 3 Adjust the pH value to 7.5-8, filter, wash the filter cake with water, and dry to obtain compound IV as the product. The product is weighed and the content of compound IV is detected by HPLC.
[0029] HPLC conditions:
[0030] Use octadecylsilane bonded silica gel as the stationary phase; use 0.05mol / L phospha...
Embodiment 5
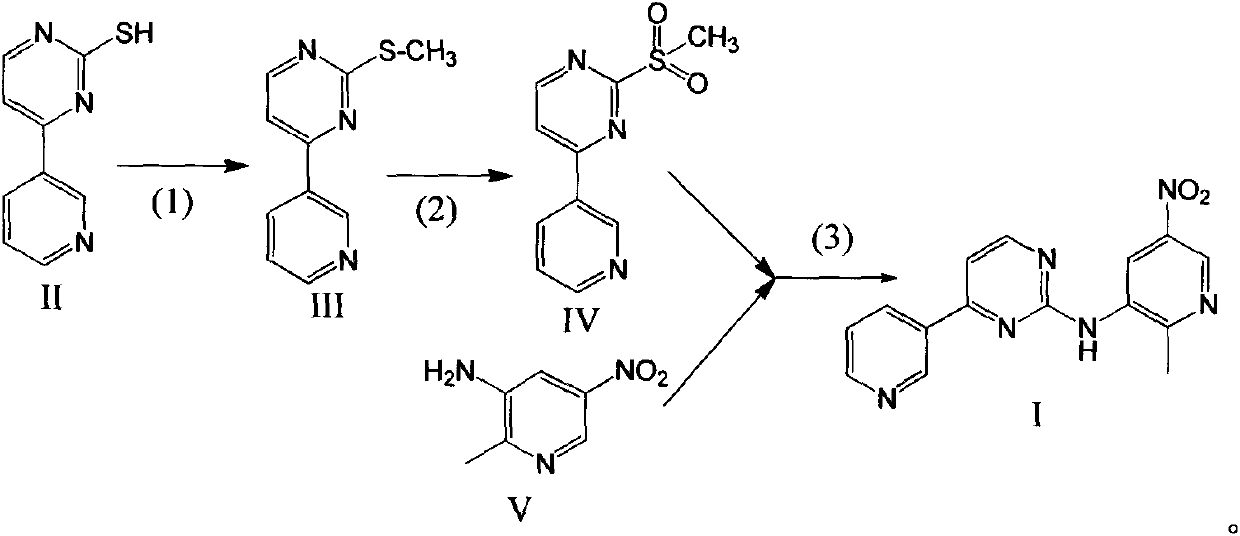
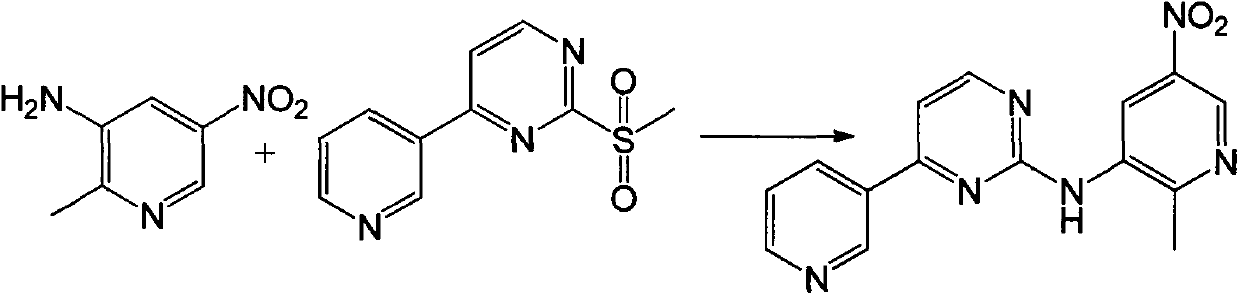
[0046] The preparation of embodiment 5 compound I
[0047] Take the compound IV prepared by the method of Example 1 as a raw material,
[0048] The preferred process of the step (3) is as follows, adding compound IV and compound V with a molar ratio of 1:1.5 into the reactor, adding DMF with a volume weight ratio of 8 to compound IV, stirring and dissolving, cooling to -10°C, and keeping at Add sodium hydride in batches at this temperature, the molar ratio of added sodium hydride to compound IV is 2:1, keep the temperature at 20-30° C. until the reaction is complete to obtain compound I after adding sodium hydride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com