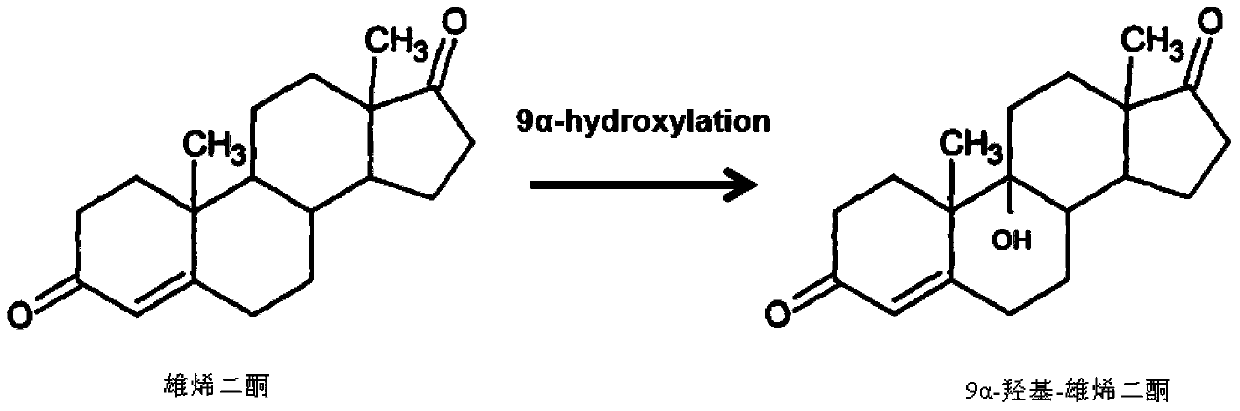
Method for preparing 9alpha-hydroxide-androstenedione by utilizing microbial conversion
A technology for microbial transformation and androstenedione, which is applied in the directions of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of low feeding concentration, long conversion time and high production cost, and achieves high feeding concentration and high production cost. Simple process and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Primary seed cultivation
[0038] Primary seed medium: peptone 0.5g / 100mL, yeast extract 1.3g / 100mL~1.4g / 100mL, glucose 1.2g / 100mL, water, adjust pH to 7.0-7.2, and sterilize.
[0039] Take an inoculation loop of Rhodococcus erythropolis (Rhodococcus erythropolis) ATCC14887 and put it in 20mL primary seed medium, and culture it at 30°C for 18h on a rotary shaker at 200rpm to obtain seeds suitable for inoculation.
[0040] (2) Secondary fermentation culture
[0041] Transformation medium: glucose 0.5g / 100mL, peptone 0.2g / 100mL, corn steep liquor 2.5g / 100mL, dipotassium hydrogen phosphate 0.02g / 100mL, magnesium sulfate 0.01g / 100mL, water, initial pH 6.8, and sterilized.
[0042] Inoculate 5 mL of the seeds obtained in step (1) into 95 mL of transformation medium [the inoculum size is 5% (V / V)], and shake at 200 rpm for 18 hours at 28°C to obtain a fermentation broth.
[0043] (3) Conversion of the substrate androstenedione
[0044] Dissolve 2 g of androstenedione wit...
Embodiment 2
[0050] (1) Primary seed cultivation
[0051] The steps were the same as in Example 1, but the culture conditions were changed to: culture at 35° C. for 12 hours on a rotary shaker at 150 rpm.
[0052] (2) Secondary fermentation culture
[0053] Secondary transformation medium: glucose 1g / 100mL, peptone 0.6g / 100mL, corn steep liquor 3.5g / 100mL, dipotassium hydrogen phosphate 0.06g / 100mL, magnesium sulfate 0.03g / 100mL, water, initial pH 7.0, and sterilized .
[0054] Inoculate 5 mL of the seeds obtained in step (1) into 95 mL of transformation medium [the inoculum size is 5% (V / V)], and culture at 30°C for 20 h with shaking at 250 rpm to obtain a fermentation broth.
[0055] (3) Conversion of the substrate androstenedione
[0056] At 90°C, dissolve 3.5g of androstenedione with 3mL of methanol, put it into about 100mL of the fermentation broth obtained in step (2) under sterile conditions, and continue to shake at 250rpm at 30°C for 56h to obtain the transformation liquid . ...
Embodiment 3
[0062] (1) Primary seed cultivation
[0063] The steps are the same as in Example 1, but the culture conditions are changed: culture at 26° C. for 15 h on a rotary shaker at 180 rpm.
[0064] (2) Secondary fermentation culture
[0065] Secondary transformation medium: glucose 1.5g / 100mL, peptone 1g / 100mL, corn steep liquor 5.0g / 100mL, dipotassium hydrogen phosphate 0.08g / 100mL, magnesium sulfate 0.05g / 100mL, water, initial pH 7.5, and sterilized .
[0066] Inoculate 5 mL of the seeds obtained in step (1) into 95 mL of transformation medium [the inoculum size is 5% (V / V)], and shake at 280 rpm for 16 hours at 32°C to obtain a fermentation broth.
[0067] (3) Conversion of the substrate androstenedione
[0068] At 85°C, dissolve 4.5g of androstenedione with 5mL of methanol, put it into about 100mL of the fermentation broth obtained in step (2) under sterile conditions, and continue to shake at 280rpm at 32°C for 72h to obtain the transformation liquid .
[0069] Among them,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com