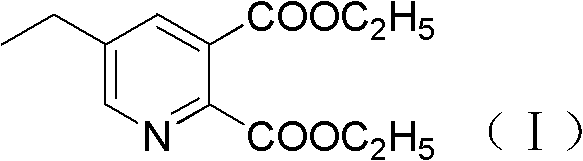
The preparation method of 5-ethylpyridine-2,3-dicarboxylate diethyl ester
A technology of diethyl diformate and diethyl chlorooxaloacetate, applied in the direction of organic chemistry and the like, can solve the problems of low yield of closed ring nitrogen sources, many three wastes, and high production cost, and achieves easy industrialization and application. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
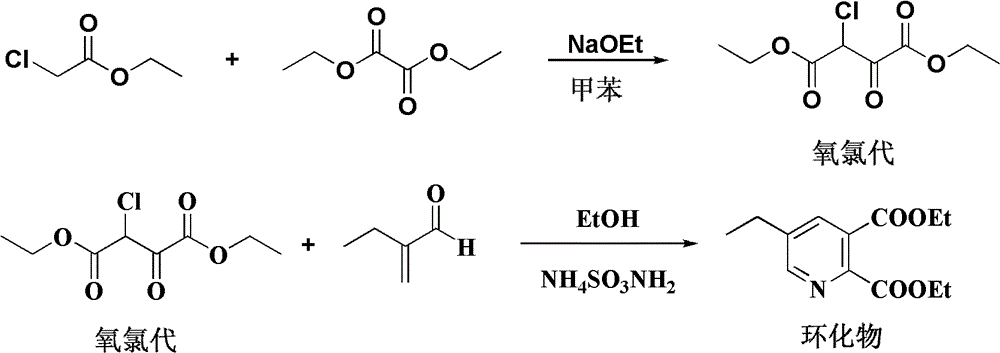
[0034] Preparation of diethyl α-chlorooxaloacetate (referred to as oxychloro)
[0035] Add 15g of sodium ethoxide to 500g of toluene, stir evenly, raise the temperature to 40-45°C, add dropwise a mixture of 112.5g (0.75mol) of diethyl oxalate and 64.5g (0.5mol) of ethyl chloroacetate, wash after the reaction, and remove After dissolving, 108 g of oxychloride was obtained, and the analysis content was 85.6%.
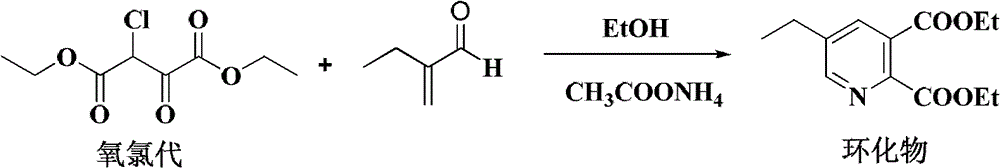
[0036] Preparation of diethyl 5-ethylpyridine-2,3-dicarboxylate
[0037] Add 60g of ethanol and 19.4g (99%, 0.25mol) of ammonium acetate into a 500ml four-necked bottle, stir and raise the temperature to ethanol 75-80°C, after the reflux is stable, add 27g (85.6%, 0.1mol) of α-chlorooxalyl dropwise A mixed solution of diethyl acetate and 12.1g (97%, 0.14mol) 2-ethylacrolein, the reaction system is controlled to be in a reflux state during the dropwise addition, and after about 30 minutes of completion of the dropwise addition, it is kept at 75-80°C for 5-6 Hours to the ...
Embodiment 2
[0039] Add 53g of ethanol and 17.2g (99%, 0.22mol) of ammonium acetate into a 500ml four-necked bottle, stir and raise the temperature to ethanol 75-80°C, after the reflux is stable, add 27g (85.6%, 0.1mol) of α-chlorooxalyl dropwise A mixed solution of diethyl acetate and 8.7g (97%, 0.10mol) 2-ethylacrolein, the reaction system is controlled to be in a reflux state during the dropping process, and after about 30 minutes of dropping, keep warm at 75-80°C for 5-6 Hour to analysis showed complete reaction of diethyl α-chlorooxaloacetate. Aftertreatment is the same as example 1, obtains brownish yellow oily liquid 26.5g, and content (gas spectrum analysis) is 87.6%, 1HNMR and IR analysis confirmed its structure, and the yield was 92.5%.
Embodiment 3
[0041] Add 73g of ethanol and 15.6g (99%, 0.2mol) of ammonium acetate into a 500ml four-neck bottle, stir and heat up to 75-80°C of ethanol, after the reflux is stable, add 27g (85.6%, 0.1mol) of α-chlorooxalyl dropwise Mixed solution of diethyl acetate and 8.7g (97%, 0.10mol) 2-ethylacrolein, control the reaction system to reflux state during the dropwise addition process, after about 30min dropwise addition, keep warm at 75-80°C for 5-6 hours until the reaction is complete. Aftertreatment is the same as example 1, obtains brownish yellow oily liquid 25.8g, and content (gas spectrum analysis) is 90.6%, 1 HNMR and IR analysis confirmed its structure, and the yield was 91.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com