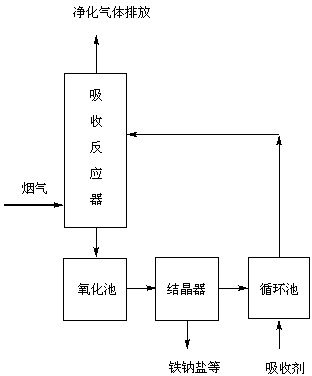
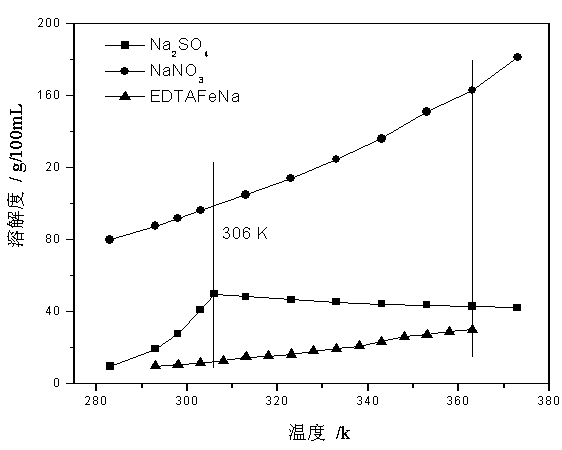
Method for producing EDTA ferric sodium salt by recovering Fe<II>EDTA wet-process complexed denitrated waste-liquid
A technology for denitrification and waste liquid, which is applied to the preparation of alkali metal nitrates, chemical instruments and methods, and organic compounds. It can solve the problems of high recycling costs, achieve low investment and operating costs, realize resource utilization, and high economic value. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Using Fe II EDTA and Na 2 SO 3 The absorption liquid is denitrified, and the waste liquid after denitrification is discharged into the oxidation tank, and hydrogen peroxide is used as the oxidant. At a reaction temperature of 15°C, the concentration of hydrogen peroxide is 0.1 M, the pH value is 4, and the oxidation time is 24 hours. The yield of FeNaEDTA is 60- 70%.
Embodiment 2
[0025] Using Fe II EDTA and Na 2 SO 3 The absorption liquid is denitrified, and the waste liquid after denitrification is discharged into the oxidation tank, and hydrogen peroxide is used as the oxidant. At a reaction temperature of 20 ° C, the concentration of hydrogen peroxide is 0.3 M, the pH value is 5, and the oxidation time is 36 hours. The yield of FeNaEDTA is 60- 78%.
Embodiment 3
[0027] Using Fe II EDTA and Na 2 SO 3 The absorption liquid is denitrified, and the waste liquid after denitrification is discharged into the oxidation tank, and hydrogen peroxide is used as the oxidant. At a reaction temperature of 25°C, the concentration of hydrogen peroxide is 0.5 M, the pH value is 3, and the oxidation time is 48 hours. The yield of FeNaEDTA is 60- 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com