Hydrophobic peptide-modified long-circulation liposome drug delivery system for injection
A long-circulating liposome and hydrophobic technology, which can be used in liposome delivery, antineoplastic drugs, drug combination, etc., can solve the problem of poor ability to enter cells, achieve improved therapeutic index, multiple designs, and wide sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the synthesis of guiding compound
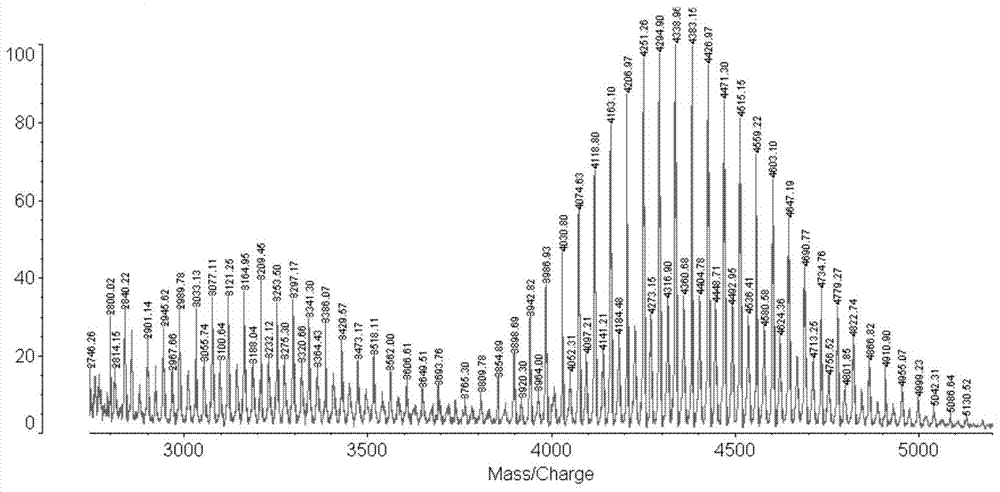
[0034] Take a certain molar ratio of DSPE-PEG-NHS and hydrophobic peptides (PFVYLI, VPTLQ, QLPVM, VTVLALGALAGVGVG, and PIEVCMYREP), use anhydrous DMF as a solvent, adjust the pH to about 8, react, use TLC to track the reaction, and use HPLC to quantify Measure unreacted ligand. After the reaction was completed, dialyzed and lyophilized, the structure of the targeting compound was confirmed using MALDI-TOF to obtain DSPE-PEG-PFVYLI, DSPE-PEG-VPTLQ, DSPE-PEG-QLPVM, DSPE-PEG-VTVLALGALAGVGVG and DSPE-PEG-PIEVCMYREP.
Embodiment 2
[0035] Embodiment 2, the synthesis of guiding compound
[0036] After epoxidizing the double bond in cyclosporine A (CsA), it reacts with ethylenediamine to obtain aminated cyclosporine A. Take a certain molar ratio of NHS-PEG-DSPE and aminated cyclosporin A, use anhydrous DMF as a solvent, adjust the pH to about 8, react, use TLC to track the reaction, and use HPLC to quantitatively measure the unreacted ligand. After the reaction was completed, dialyzed and freeze-dried, the structure of the targeting compound was confirmed using MALDI-TOF to obtain DSPE-PEG-CsA.
Embodiment 3
[0037] Embodiment 3, the cyclosporin A modified long circulation liposome of encapsulating doxorubicin
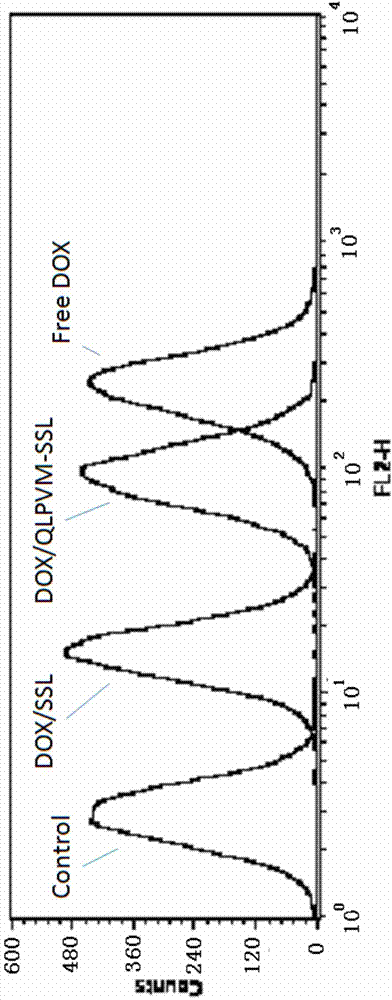
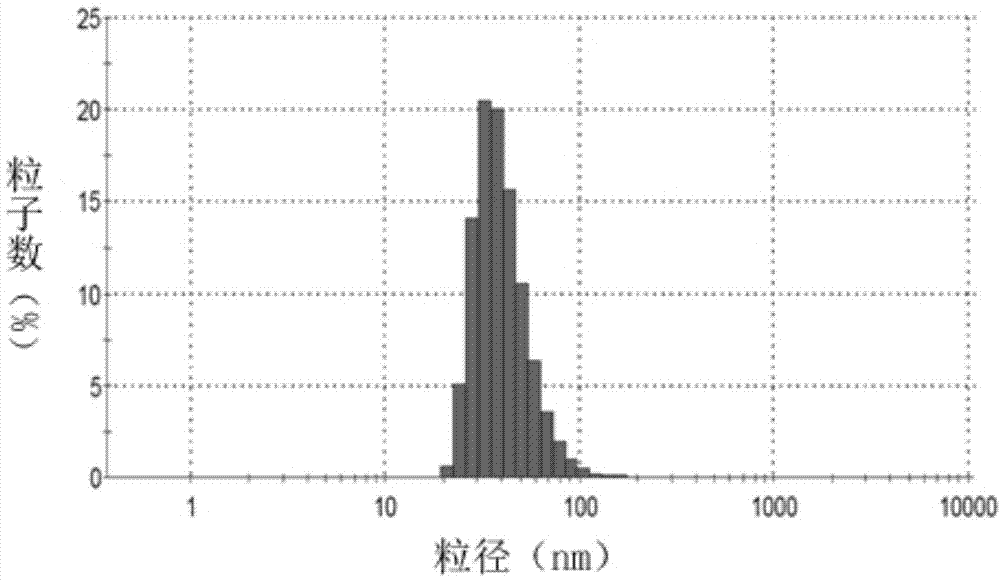
[0038] Doxorubicin is a commonly used anti-tumor drug, and its target is located in the nucleus, which is a substrate of P-gp. Take lecithin, cholesterol, DSPE-PEG, DSPE-PEG-CsA (mass ratio: 10:2.5:1.8:1), put them in an eggplant-shaped bottle and add appropriate amount of chloroform to dissolve them. Rotary evaporation under reduced pressure at 40°C formed a uniform transparent film. Add 123mM ammonium sulfate solution, and sonicate in a water bath until blue opalescence appears. The obtained liposomes were repeatedly squeezed through a 0.2 μm polycarbonate membrane, and the prepared blank liposomes were passed through a Sephadex column, eluted with PBS (pH 7.4) buffer, and the liposome fraction was collected. Preheat the liposomes in a water bath at 40°C for a while, add an appropriate amount of adriamycin aqueous solution (2 mg / ml), place on an air shaker at 40°C for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com