Pharmaceutical composition containing calcium folinate and fluorouracil
A technology of calcium folinate and fluorouracil, which is applied in the field of medicine, can solve the problems of hard shell and brittleness, shrinkage of freeze-dried powder injection, poor water solubility, etc., and achieve the effects of quick onset, improved water solubility, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Preparation of Calcium Folinate Freeze-dried Powder for Injection
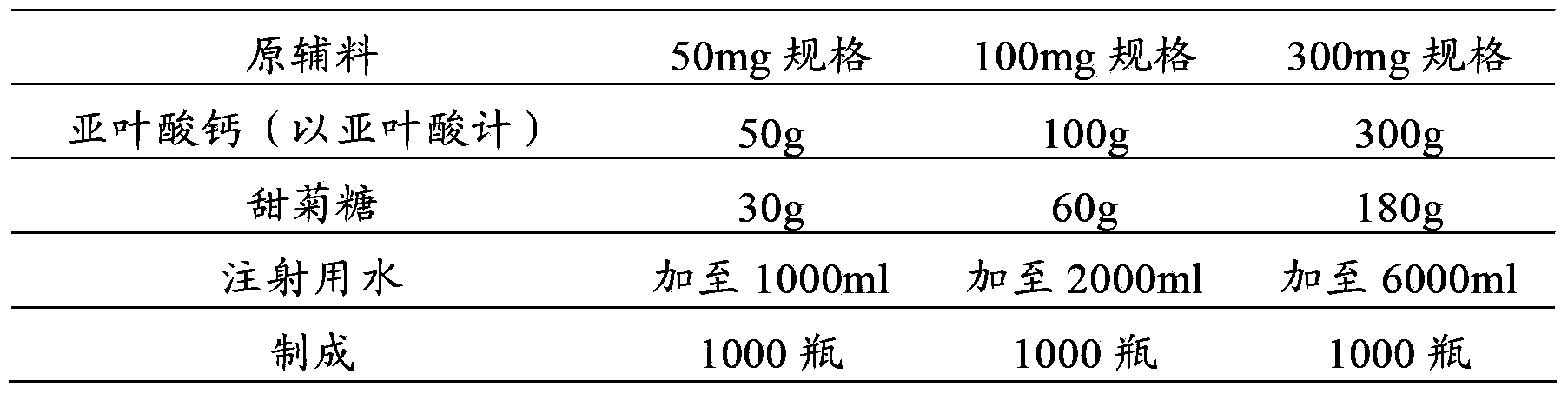
[0041] prescription:
[0042]
[0043] making process:
[0044] (1) First add 7200ml of water for injection into the container;
[0045] (2) Add 450g of calcium folinate (calculated as folinic acid) and 270g of stevioside, stir to dissolve completely, and adjust the pH to 7.5 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0046] (3) Add 2.7g of activated carbon for injection, add the remaining water for injection, set the volume to 9000ml, stir and absorb for 30 minutes;
[0047] (4) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0048] (5) Filling, 1ml / bottle or 2ml / bottle or 6ml / bottle;
[0049] (6) Freeze drying
[0050] ① During the pre-freezing period, lower the shelf temperat...
Embodiment 2
[0053] Example 2 Preparation of Calcium Folinate Injection
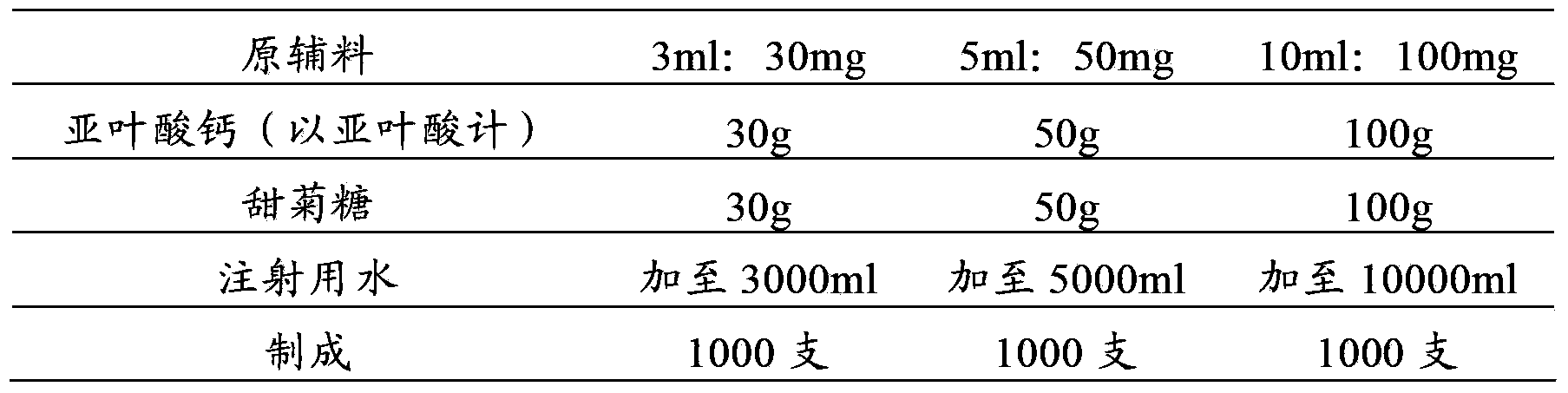
[0054] prescription:
[0055]
[0056] making process:
[0057] (1) First add 14400ml water for injection into the liquid mixing tank;
[0058] (2) Add 180g of calcium folinate (calculated as folinic acid) and 180g of stevioside, stir to dissolve completely, and adjust the pH to 7.7 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0059] (4) Add 5.4g of activated carbon for injection, add the remaining water for injection, set the volume to 18000ml, stir and absorb for 30 minutes;
[0060] (5) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0061] (6) Filling, 3ml / bottle or 50ml / bottle or 10ml / bottle, seal, sterilize to obtain calcium folinate injection.
Embodiment 3
[0062] Example 3 Preparation of Fluorouracil Injection
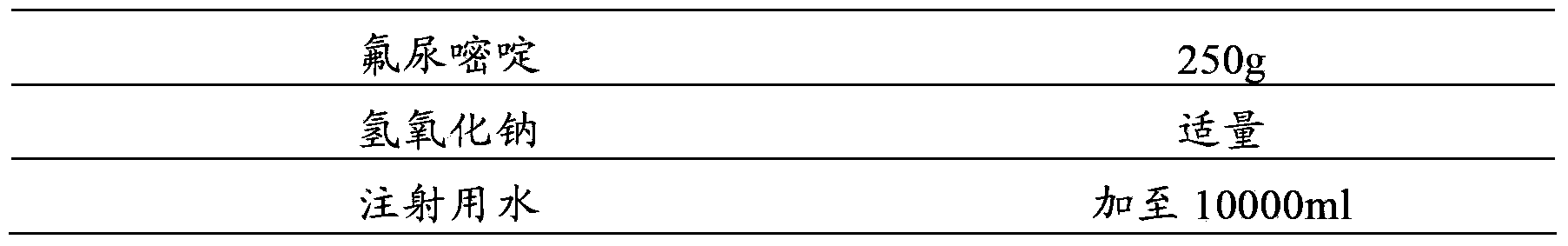
[0063] prescription:
[0064]
[0065] making process:
[0066] (1) First add 8000ml of water for injection into the container;
[0067] (2) Add 250g of fluorouracil, stir to dissolve completely, and adjust the pH to 8.7 with 1mol / L sodium hydroxide solution;
[0068] (3) Add 5g of activated carbon for injection, add the remaining water for injection, constant volume, stir and absorb for 30 minutes;
[0069] (4) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0070] (5) Filling, 10ml / bottle, sealing, and sterilizing to obtain fluorouracil injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com