New zwitter-ionic polymer containing multiple phosphonic acid end groups, preparation method and application thereof
A zwitterionic and polymer technology, applied in the field of zwitterionic polymers, can solve problems such as difficult to find groups and complex surface modification methods, achieve low concentration requirements, excellent anti-bacterial adhesion and surface lubrication, and film-forming fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Functionalized self-assembly of titanium oxide surface
[0036] (1) Preparation of polymer
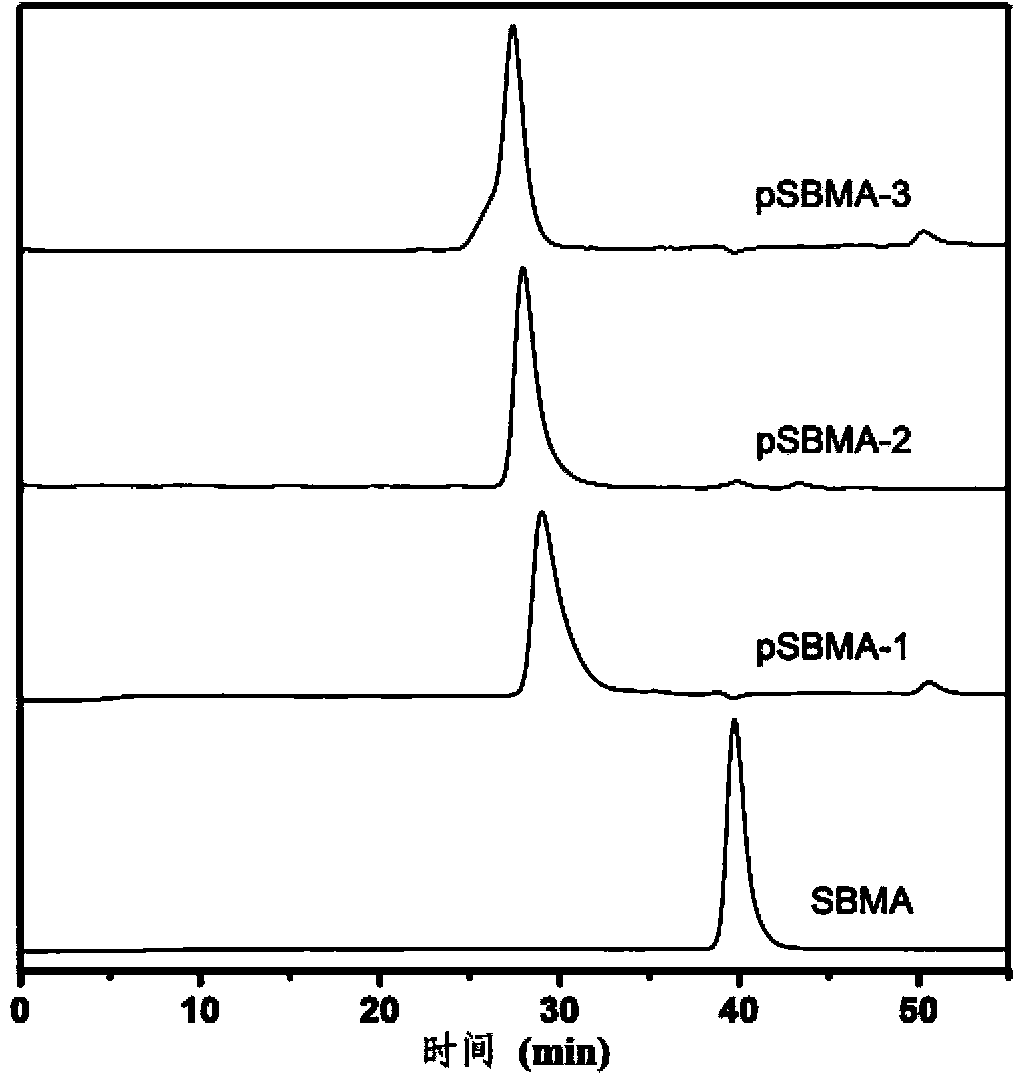
[0037] 1) Using reversible addition-fragmentation chain transfer (RAFT) polymerization method, take a 10mL eggplant-shaped reaction flask with a branch tube and add 4-cyanovaleric acid dithiobenzoic acid, 4,4' -Azobis (4-cyanovaleric acid), monomeric thiobetaine methyl acrylate (SBMA). Then add a 10:1 (volume ratio) mixture of methanol and deionized water, put in a stir bar, and quickly dissolve the solids, seal the reaction flask, and let nitrogen gas flow for 30 minutes under ice bath conditions. The anaerobic reaction system was placed in an oil bath at 60°C to obtain a polymer (named pSBMA). Pay attention to the use of tin foil to avoid light during the entire operation.
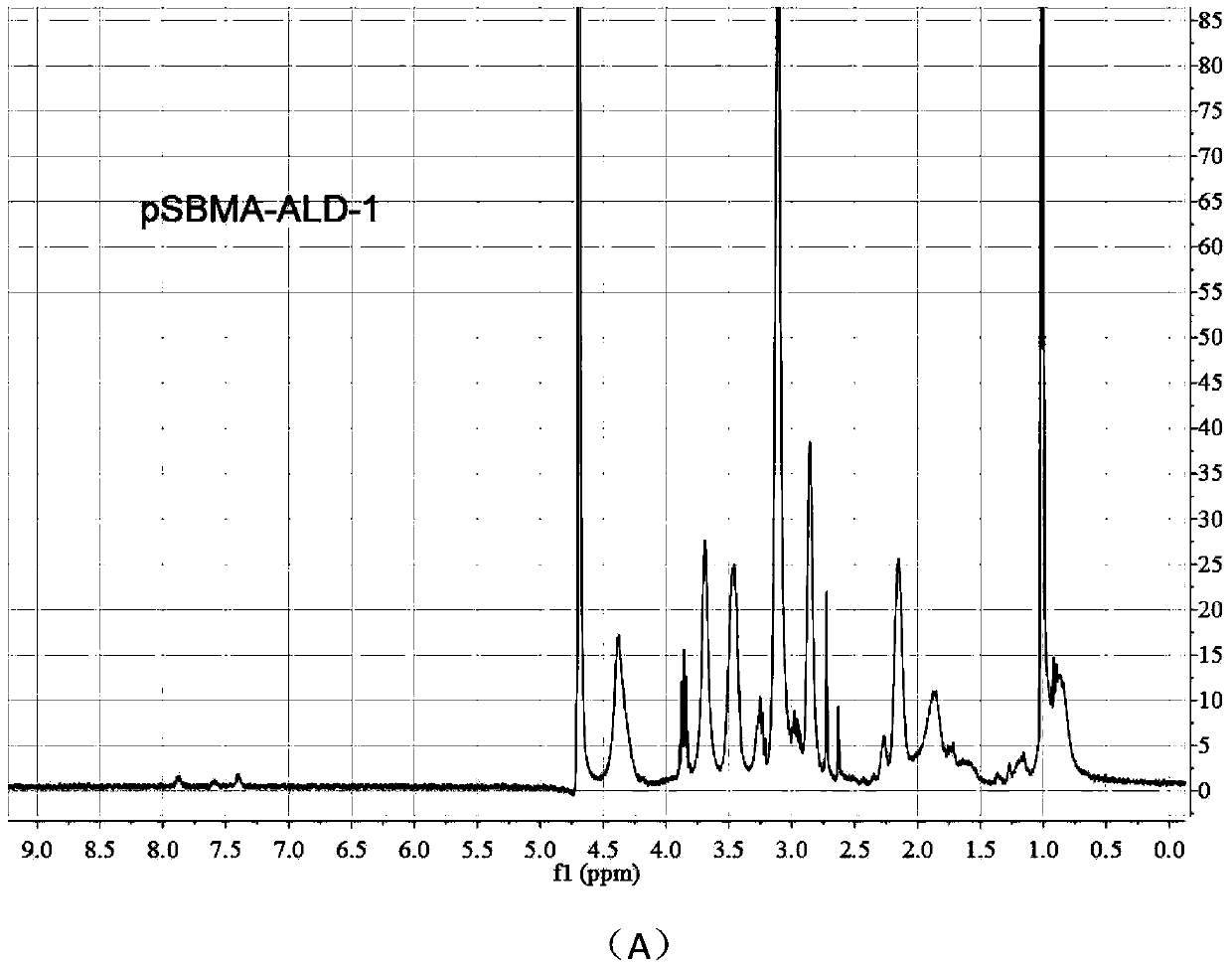
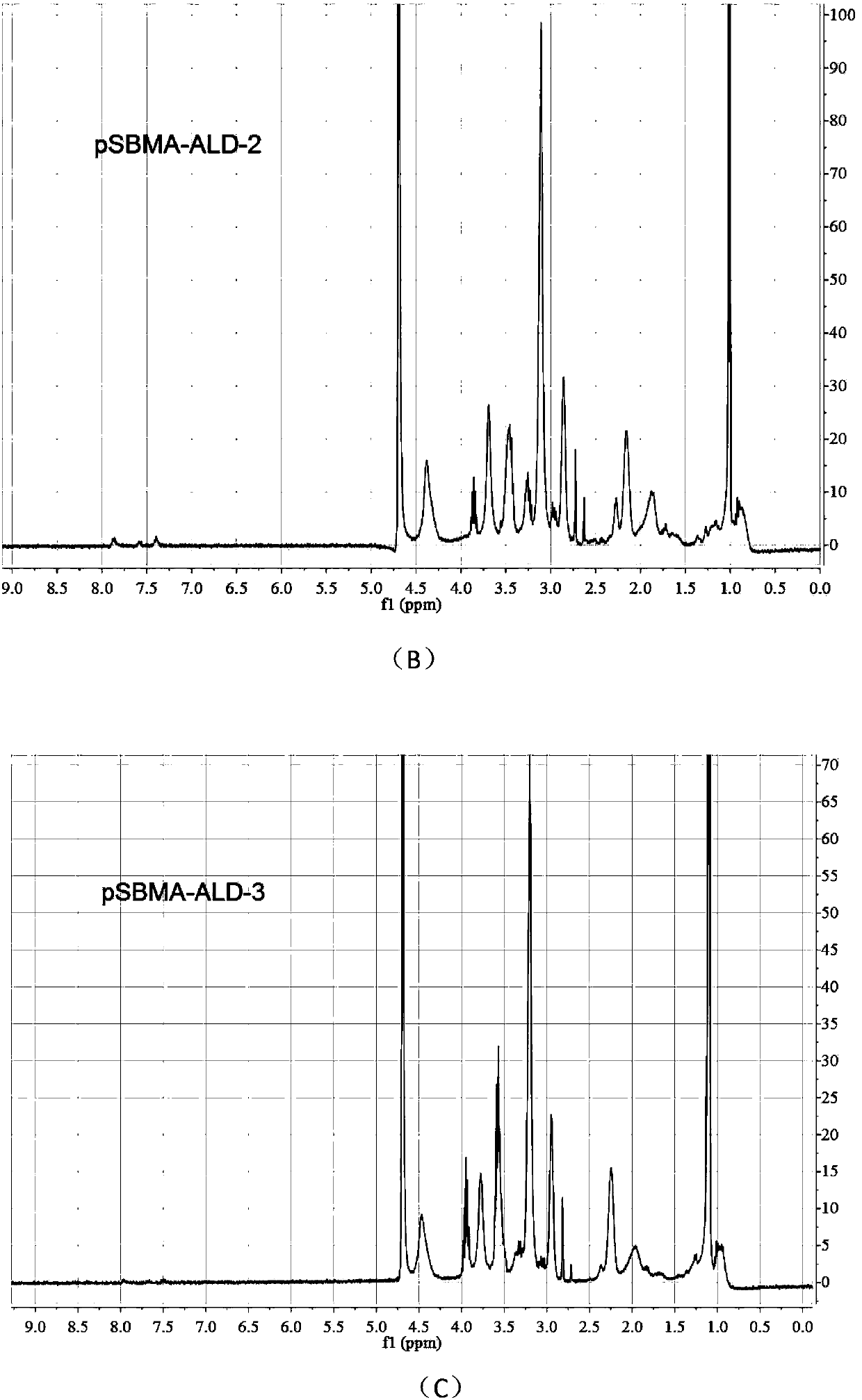
[0038] 2) Adopt the condensation and acylation reaction of carboxylic acid and amine, and take 1mL centrifuge tube as the reaction vessel. First, dissolve pSBMA in 2-(N-morpholine) ethanesulfonic a...
Embodiment 2
[0052] Example 2: Functionalized self-assembly of stainless steel surface
[0053] (1) Preparation of polymer
[0054] 1) Using reversible addition-fragmentation chain transfer (RAFT) polymerization method, take a 10mL eggplant-shaped reaction flask with a branch tube and add 4-cyanovaleric acid dithiobenzoic acid, 4,4' -Azobis (4-cyanovaleric acid), monomeric thiobetaine methyl acrylate (SBMA). Then add a 10:1 (volume ratio) mixture of methanol and deionized water, put in a stir bar, and quickly dissolve the solids, seal the reaction flask, and let nitrogen gas flow for 30 minutes under ice bath conditions. The anaerobic reaction system was placed in an oil bath at 60°C to obtain a polymer (named pSBMA). Pay attention to the use of tin foil to avoid light during the entire operation.
[0055] 2) Adopt the condensation and acylation reaction of carboxylic acid and amine, and take 1mL centrifuge tube as the reaction vessel. First, dissolve pSBMA in 2-(N-morpholine) ethanesulfonic ...
Embodiment 3
[0070] Example 3: Fe 3 O 4 Surface modification of magnetic nanoparticles
[0071] (1) Preparation of polymer
[0072] 1) Using reversible addition-fragmentation chain transfer (RAFT) polymerization method, take a 10mL eggplant-shaped reaction flask with a branch tube and add 4-cyanovaleric acid dithiobenzoic acid, 4,4' -Azobis (4-cyanovaleric acid), monomeric thiobetaine methyl acrylate (SBMA). Then add a 10:1 (volume ratio) mixture of methanol and deionized water, put in a stir bar, and quickly dissolve the solids, seal the reaction flask, and let nitrogen gas flow for 30 minutes under ice bath conditions. The anaerobic reaction system was placed in an oil bath at 60°C to obtain a polymer (named pSBMA). Pay attention to the use of tin foil to avoid light during the entire operation.
[0073] 2) Adopt the condensation and acylation reaction of carboxylic acid and amine, and take 1mL centrifuge tube as the reaction vessel. First, dissolve pSBMA in 2-(N-morpholine) ethanesulfonic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com