Preparation method of diamino diphenyl ether
A technology of diaminodiphenyl ether and aromatic ether, which is applied in the field of preparation of fine chemicals, can solve the problems of not meeting the demand for nitration of diphenyl ether, and achieve the effect of less three wastes, low cost, and reduced loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
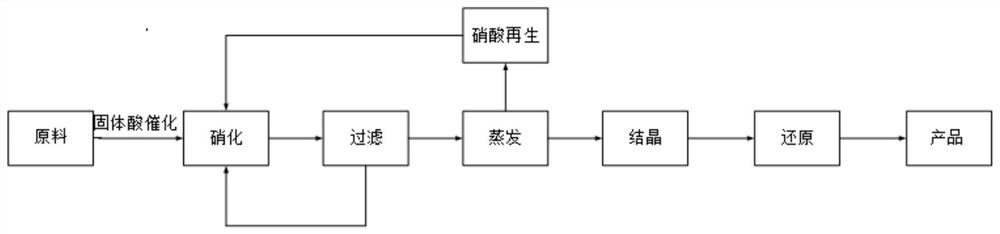
[0035] (1) Nitrification reaction
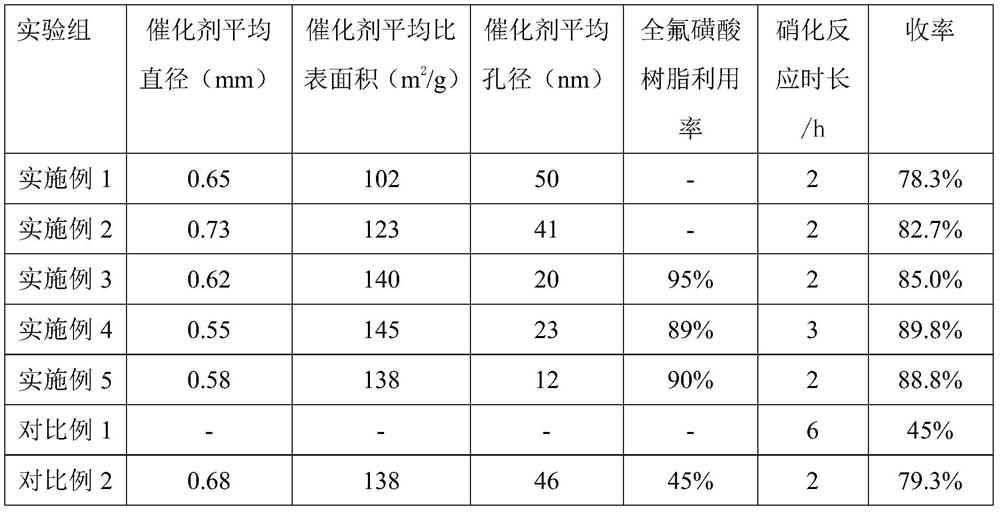
[0036] With diphenyl ether as reaction substrate, with 70wt% nitric acid as nitrating agent and solvent, with perfluorosulfonic acid resin as catalyst, wherein the mass ratio of diphenyl ether, nitric acid and perfluorosulfonic acid resin is 1:10:0.1, The reaction was stirred at 30° C. and 1 atm for 0.5 h, at 40° C. and 1 atm for 0.5 h, and at 50° C. and 1 atm for 1 h.
[0037] (2) hot filter
[0038] Centrifuge the mixture obtained in step (1) at 65°C, recover the perfluorosulfonic acid resin catalyst obtained from the filter for the next batch of reactions, evaporate and concentrate the obtained filtrate, and mix the evaporated nitric acid with 80% sulfuric acid Carry out distillation, collect distilled components, obtain 99wt% concentrated nitric acid, pass air into it, continue 5min, obtain the nitric acid after regeneration, be used for the next batch of nitration reaction.
[0039] (3) Catalytic hydrogenation
[0040] Dilute the pro...
Embodiment 2
[0043] (1) Nitrification reaction
[0044] With diphenyl ether as the reaction substrate, with 70wt% nitric acid as nitrating agent and solvent, with perfluorosulfonic acid resin as catalyst, wherein the mass ratio of diphenyl ether, nitric acid and perfluorosulfonic acid resin is 1:10:0.1, The reaction was stirred at 30° C. and 1 atm for 0.5 h, at 40° C. and 1 atm for 0.5 h, and at 50° C. and 1 atm for 1 h.
[0045] (2) hot filter
[0046] Centrifuge the mixture obtained in step (1) at 75°C, recover the perfluorosulfonic acid resin catalyst obtained from the filter for the next batch of reactions, evaporate and concentrate the obtained filtrate, and mix the evaporated nitric acid with 80% sulfuric acid Carry out distillation, collect distilled components, obtain 99wt% concentrated nitric acid, pass air into it, continue 5min, obtain the nitric acid after regeneration, be used for the next batch of nitration reaction.
[0047] (3) Catalytic hydrogenation
[0048] Dilute the p...
Embodiment 3
[0051] (1) Perfluorosulfonic acid resin catalyst pretreatment
[0052] Put the perfluorosulfonic acid resin in the ethanol-water solution, first stir the mixture at 55°C for 2h, then stir and dissolve it at 135°C and 35MPa for 40 hours in an argon atmosphere, and extract the solution with carbon tetrachloride. The extract from the lower layer was added to the sodium silicate solution, aged for 1.5 hours, dried at 95°C for 24 hours, and ground to obtain a perfluorosulfonic acid resin catalyst.
[0053] (2) Nitrification reaction
[0054] With diphenyl ether as reaction substrate, 99wt% nitric acid as nitrating agent and solvent, perfluorosulfonic acid resin as catalyst, wherein the mass ratio of diphenyl ether, nitric acid and perfluorosulfonic acid resin is 1:5:0.001, The reaction was stirred at 30° C. and 1 atm for 0.5 h, at 45° C. and 1 atm for 0.5 h, and at 65° C. and 1.5 atm for 1 h.
[0055] (3) hot filter
[0056] Centrifuge the mixture obtained in step (1) at 70°C, r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com