Inclusion compound containing celecoxib and preparation method thereof
A celecoxib and inclusion compound technology, applied in the field of celecoxib inclusion compounds, can solve the problems of difficult micronization, difficult pulverization, and low bulk density of celecoxib raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
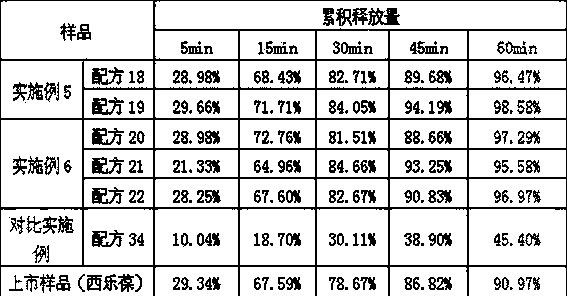
Embodiment 1
[0015] Recipe composition:
[0016]
[0017] The raw material of celecoxib is self-made by our company, and the approval number is: Guoyao Zhunzi H20133228.
[0018] Preparation method: Weigh the formulated amount of celecoxib, add an appropriate amount of ethanol to dissolve it completely, keep a certain temperature, add the formulated amount of the saturated aqueous solution of the inclusion carrier dropwise, continue stirring for 1 hour, stop heating, and continue stirring to room temperature , to obtain a white suspension, which can be obtained by vacuum drying after filtration, or the white suspension can be directly freeze-dried.
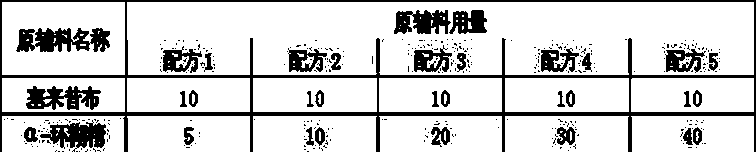
Embodiment 2
[0020] Recipe composition:
[0021]
[0022] Preparation method: Weigh the formulated amount of celecoxib, add an appropriate amount of ethanol to dissolve it completely, keep a certain temperature, add the formulated amount of the saturated aqueous solution of the inclusion carrier dropwise, continue stirring for 1 hour, stop heating, and continue stirring to room temperature , to obtain a white suspension, which can be directly freeze-dried.
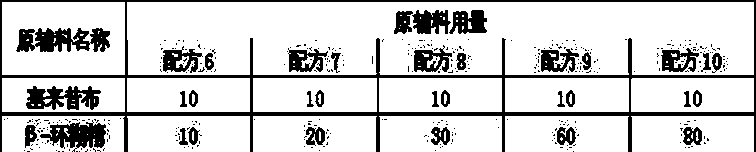
Embodiment 3
[0024] Recipe composition:
[0025]
[0026] Preparation method: Weigh the formulated amount of celecoxib, add an appropriate amount of ethanol to dissolve it completely, keep a certain temperature, add the formulated amount of the saturated aqueous solution of the inclusion carrier dropwise, continue stirring for 1 hour, stop heating, and continue stirring to room temperature , to obtain a white suspension, which was obtained by vacuum drying after filtration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com