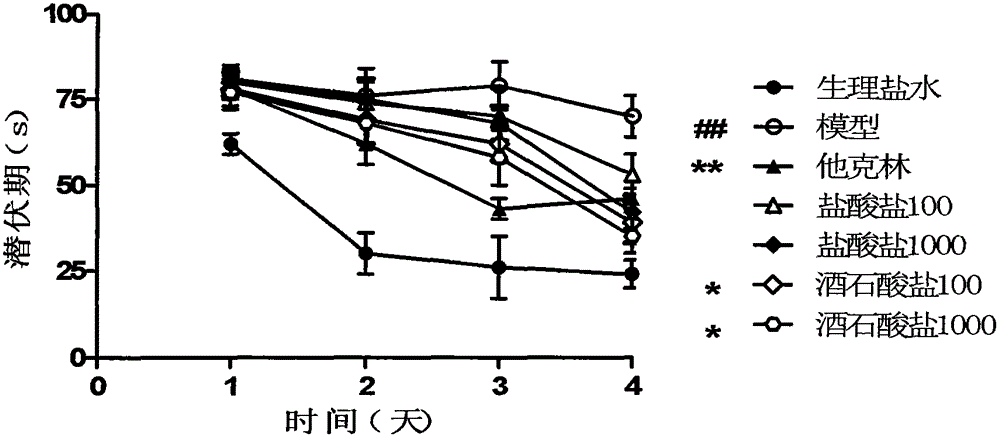
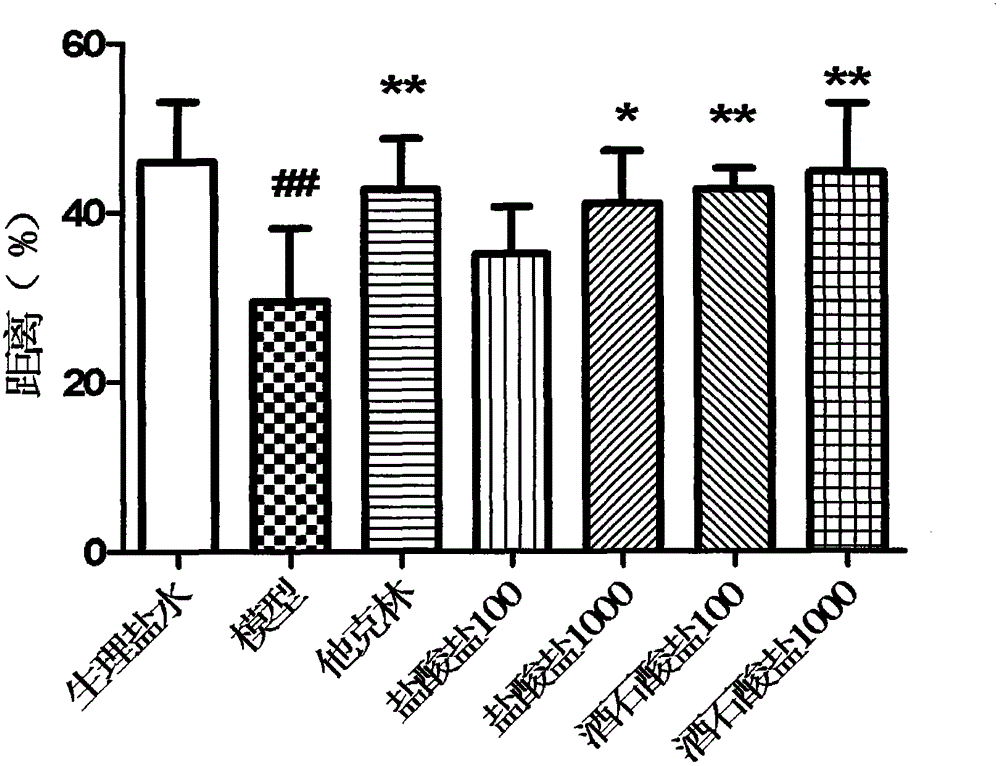
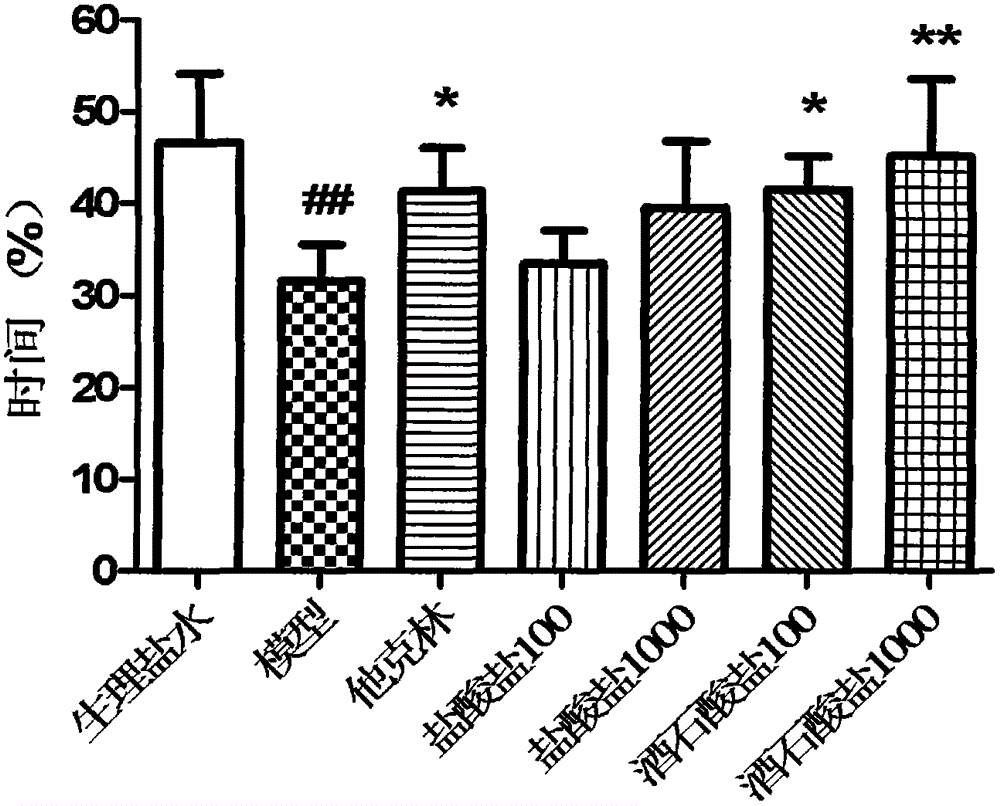
A kind of levomaprotamol phenylcarbamate-l-(+)-tartrate and its preparation method
A technology of meprotamol phenylcarbamate and phenol phenylcarbamate, applied in the field of levomaprotamol phenylcarbamate-L--tartrate and its preparation, can solve the difficulty of industrial production and control, HCl-anhydrous ether is difficult to prepare and store, and is not suitable for industrial production, etc., to achieve the effects of low cost, shortened reaction time, and improved work efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 levomabutamol
[0032] 2.0Kg (8.58mol) racemic mebutamol and 3.07Kg (8.58mol) L-(-)-dibenzoyl tartaric acid (L-(-)-DBTA) were placed in a 100.0L reactor, and no Water ethanol 47.3L (37.4Kg), after heating and reflux for 3.0 hours, stop heating, turn on condensed water to cool, wait until the temperature drops to 8.8°C, release the white solid suspension from the bottom of the kettle, suction filter, and wash with 1.1kg of ethanol Filter cake to obtain white powder levomaprotamol-L-(-)-dibenzoyl tartrate, wet weight 3.70Kg, dry in an infrared drying oven at 40-42°C overnight, dry weight 2.30Kg, yield: 90.6 %.
[0033] Add 2.30Kg of levomaprotamol-L-(-)-dibenzoyl tartrate into the reaction kettle, add 25.6L (20.3Kg) of methanol, and keep the temperature at 60°C for 2.0 hours. Stop heating, turn on the condensate water, and when the temperature drops to 8.8°C, release the white solid suspension from the bottom of the kettle, filter it with...
Embodiment 2
[0035] The synthesis of embodiment 2 levomaprotamol phenylcarbamate
[0036] Levomaprotamol (100 g, 0.429 mol) was dissolved in 3000 mL of ethyl acetate, phenyl isocyanate (61.26 g, 0.514 mol) was added, and stirred overnight at room temperature. TLC detects that the starting material disappears.
[0037] Add 1000mL of water to destroy the remaining unreacted phenyl isocyanate, adjust the pH to 3 with 1N hydrochloric acid, separate the layers, and extract the aqueous phase with 500mL×2 ethyl acetate. Add 1500 mL ethyl acetate to the water phase, then add ammonia water dropwise to adjust the pH to 9, separate the layers, and extract the water phase with 500 mL×2 ethyl acetate. Combined organic phases, anhydrous Na 2 SO 4 dry. Filtrate and concentrate to obtain levomaprotamol phenylcarbamate, 141.0 g of off-white solid (crude product).
[0038] Put the obtained 141.0g crude product in a 3000mL beaker, add 700mL acetonitrile, heat it in a water bath to 50°C under stirring to...
Embodiment 3
[0039] Example 3 Preparation of Levomaprotamol phenylcarbamate-L-(+)-tartrate
[0040] The L-(+)-tartaric acid of 45.0g levomaprotamol phenyl carbamate and 19.2g is placed in the 1.0L single-necked bottle, add 400mL acetone, heat reflux under stirring and make solid dissolve completely, after 1.0 hour, Heating was stopped, the acetone was spin-dried under reduced pressure, and then the temperature of the water bath was kept at 50° C. for 1.0 hour under reduced pressure, and the obtained light yellow foamy solid was placed in a vacuum drying oven at room temperature and dried under reduced pressure with an oil pump overnight (P 2 o 5 is a desiccant), and finally the solid obtained is placed in a vacuum oven at 50° C. and dried under reduced pressure by an oil pump (P 2 o 5 be the desiccant) in 4.0 hours to obtain 62.1 g of levomaprotamol phenylcarbamate-L-(+)-tartrate light yellow foamy solid, [α] 25 D -11.61 (c1.013, EtOH); 1HNMR (600MHz, DMSO-d 6 )δ10.19(s, 1H, CONH), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com