Water-soluble andrographolide derivatives, preparation method thereof and application on medicine
A technology of andrographolide, derivatives, applied in antipyretics, antibacterial drugs, drug combinations and other directions, can solve the problems of complex components, difficult quality control, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] step 1:
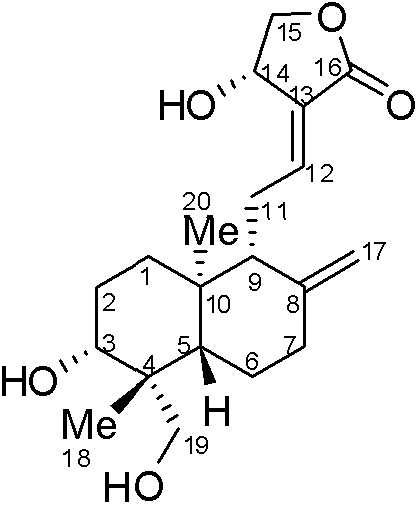
[0034] Add 1.93g of andrographolide (III), 15mL of acetone and 134mg of p-toluenesulfonic acid successively to a 50mL single-necked flask equipped with a spherical reflux condenser, react for 1 hour under stirring and reflux, cool to room temperature, and add 100mL of ethyl acetate to the system The ester was washed successively with saturated sodium bicarbonate solution and water, dried, concentrated under reduced pressure, and purified to obtain 1.99 g of white solid product II.
[0035] 1 H NMR (400MHz, CDCl 3 ): δ6.920(t, J=6.0Hz, 1H), 5.020(s, 1H), 4.892(s, 1H), 4.613(s, 1H), 4.654(s, 1H), 4.436(m, 1H) , 4.265(m, 1H), 3.950(d, J=11.7Hz, 1H), 3.477(m, 1H), 3.175(d, J=11.7Hz, 1H), 2.559(m, 2H), 2.400(m, 1H), 1.960(m, 2H), 1.750(m, 5H), 1.400(s, 3H), 1.360(s, 3H), 1.284(m, 3H), 1.195(s, 3H), 0.925(s, 3H) ); MS (ESI) m / z: 391[M+1].
[0036] Step 2:
[0037] Add 1.95g of compound II, 0.98g of sulfur trioxide triethylamine salt and 10mL of met...
Embodiment 2
[0040]
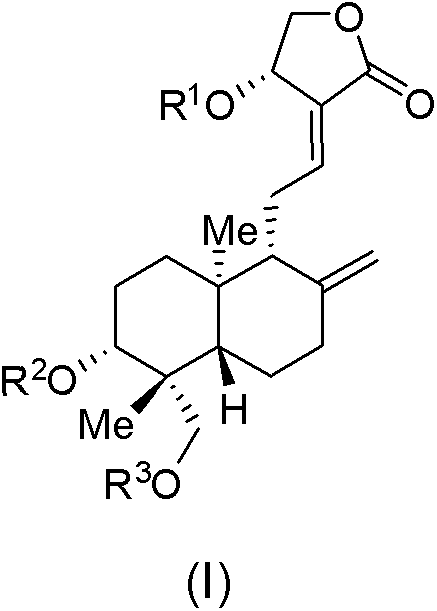
[0041] In the 50mL one-necked flask, add 1.53g compound Ia, 10g cationic resin (Amberlite IR120) and 20 mL of methanol, stirred at room temperature for 15 minutes, filtered, the solid was washed with methanol, the organic phases were combined, part of the methanol solution was taken, and concentrated under reduced pressure to obtain a white solid product Ib.
[0042] MS(ESI) m / z: 429[M-1].
[0043] The above methanol solution was washed with saturated NaHCO 3 The pH value of the solution was adjusted to pH=7, and concentrated under reduced pressure to obtain 1.15 g of white solid product Ic.
[0044] 1 H NMR (400MHz, D 2 O): δ6.988(t, J=6.4Hz, 1H), 5.608(s, 1H), 4.786(s, 1H), 4.540(d, J=3.6Hz, 2H), 4.503(s, 1H), 3.970(d, J=12.4Hz, 1H), 3.371(m, 2H), 2.560(m, 2H), 2.309(m, 1H), 1.934(m, 2H), 1.694(m, 4H), 1.222(m , 3H), 1.055(s, 3H), 0.591(s, 3H); MS(ESI) m / z: 429(M-1).
Embodiment 3
[0046]
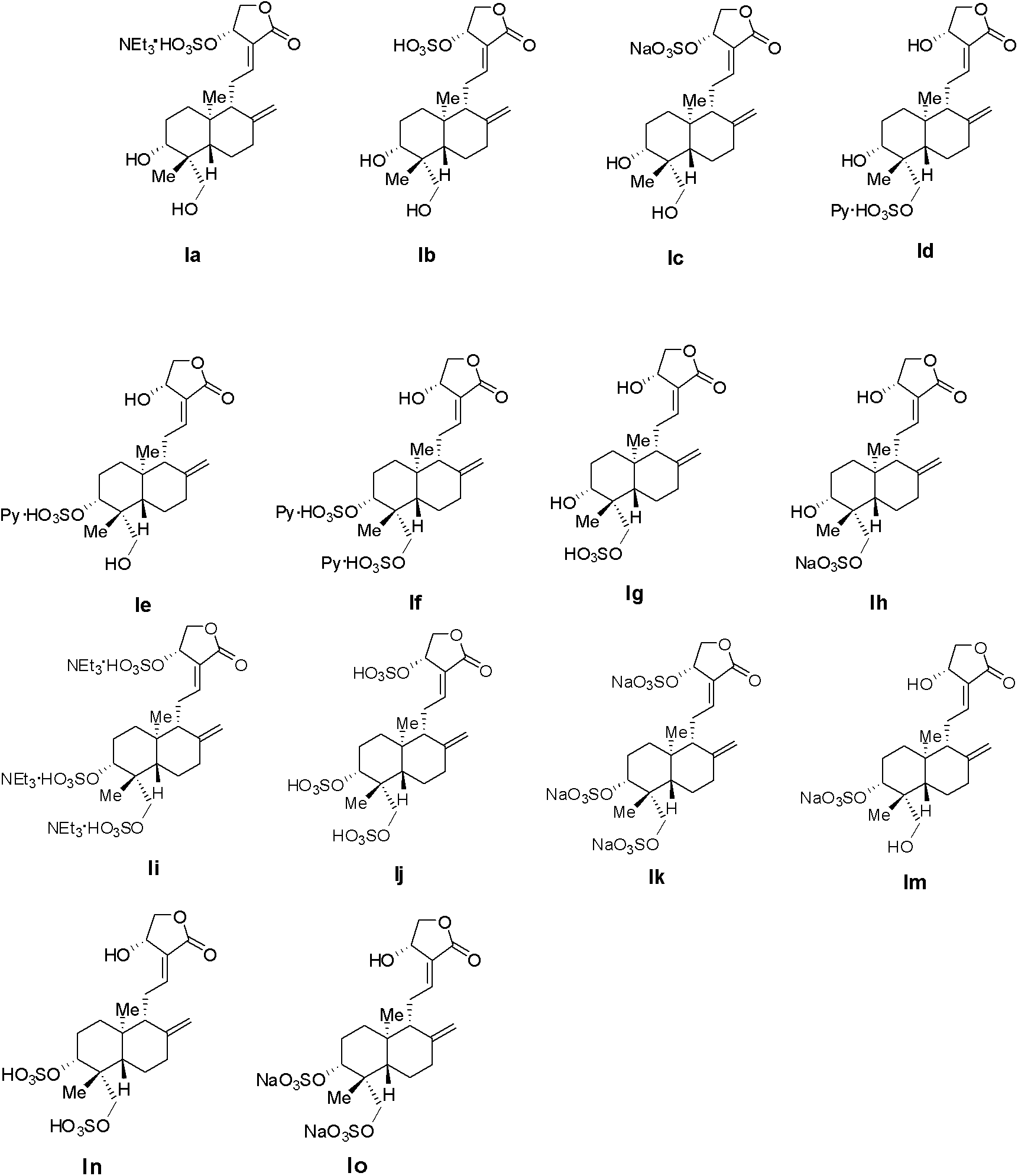
[0047] Add 42.05g of andrographolide (III) and 150mL of pyridine sequentially into a 500mL three-necked flask equipped with a thermometer, cool to zero, add 30.95g of sulfur trioxide pyridinium salt, stir to fully dissolve, rise to room temperature, react for 3 hours, and pour into the system 500 mL of ethyl acetate was added to dilute and stirred for 5 minutes to obtain white solid products Id (24.31 g), Ie (2.73 g) and If (7.34 g) after purification.
[0048] Id: 1 H NMR (400MHz, CD 3 OD): δ8.910(d, J=5.6Hz), 8.703(m, 1H), 8.156(m, 2H), 6.857(t, J=6.4Hz, 1H), 5.040(d, J=5.2Hz, 1H), 4.879(s, 1H), 4.666(s, 1H), 4.483(dd, J 1 =10.0Hz,J 2 =6.4Hz, 1H), 4.183(m, 3H), 3.323(m, 1H), 2.621(m, 2H), 2.410(m, 1H), 1.743-1.990(m, 7H), 1.325(m, 2H) , 1.205(s, 3H), 0.787(s, 3H); MS(ESI) m / z: 429[M-1].
[0049] Ie: 1 H NMR (400MHz, CD 3 OD): δ8.909(d, J=5.6Hz, 2H), 8.705(m, 1H), 8.155(m, 2H), 6.847(t, J=6.4Hz, 1H), 5.020(d, J=5.7 Hz, 1H), 4.879(s, 1H), 4.660(s, 1H), 4.46...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com