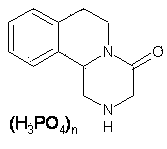
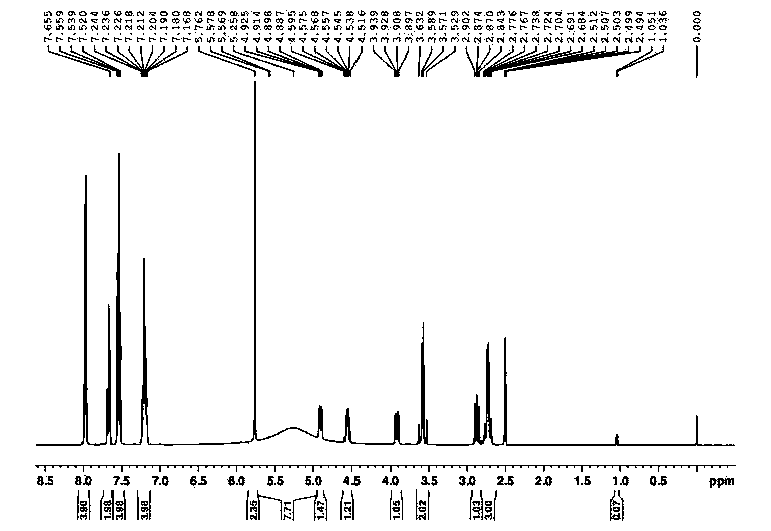
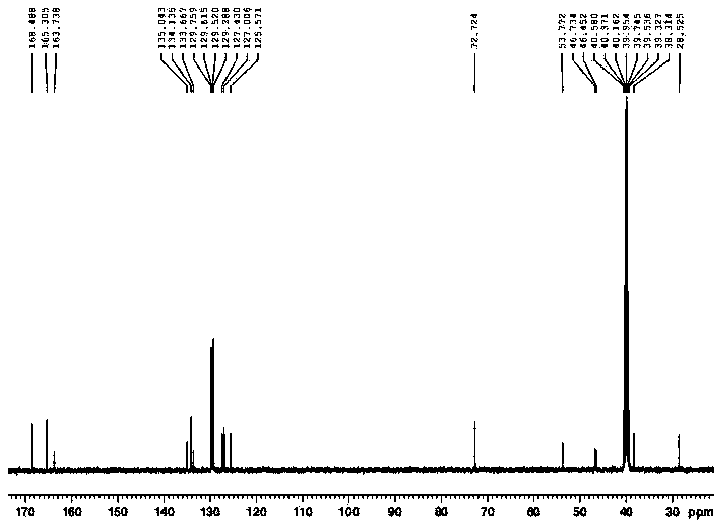
Preparation method for levo-praziquantel
A technology of L-praziquantel and praziquantel amine, which is applied in the field of preparation of antiparasitic drugs, can solve the problems of long synthetic process route, harsh reaction conditions, and high impurity content, and achieve improved yield, purity, and good stability , The effect of simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] A preparation method of L-praziquantel, carried out according to the following steps:
[0053] (1) Add 95g of praziquantel amine phosphate, 900g of isopropanol and 245g of water in sequence in a 1000mL three-necked flask equipped with a reflux condenser, a thermometer, and a magnetic stirring device, and dissolve. Add 85g of sodium hydroxide solution (30% by weight) dropwise, stir for 1 hour, cool to 10°C and filter;
[0054] (2) Add 73g of isopropanol solution of L-dibenzoyltartaric acid to the filtrate, add 200g of isopropanol, heat to dissolve and clarify, slowly cool to 20°C, continue stirring for 10h, and centrifuge to obtain L-praquine Ketoamine tartrate crude product 47 g, HPLC purity: 99.93%, ee%: 98.12%;
[0055] (3) Add 47g of crude L-praziquantel amine tartrate to a mixed solution of 506g of isopropanol and 202g of pure water, heat until completely dissolved, slowly cool to 20°C, continue to stir for 10h, and centrifuge to obtain L-praziquantel Amine tartra...
Embodiment 2
[0066] A kind of preparation method of L-praziquantel of the present embodiment is carried out according to the following steps:
[0067] (1) In a 5000mL three-neck flask equipped with a reflux condenser, a thermometer and a magnetic stirring device, add 481g of praziquantelamine phosphate, 4500g of isopropanol and 1225g of water in sequence to dissolve. Add 425g of sodium hydroxide solution (30% by weight) dropwise, stir for 1 hour, cool to 10°C and filter;
[0068] (2) Add 370g of the isopropanol solution of L-dibenzoyltartaric acid to the filtrate, add 1000g of isopropanol, heat to dissolve and clarify, slowly cool to 20°C, continue to stir for 10h, and centrifuge to obtain L-praziquine Ketoamine tartrate 47 g, HPLC purity: 99.93%, ee%: 98.12%;
[0069] (3) Add 238g of the crude product of L-praziquantel amine tartrate into a mixed solution of 2560g of isopropanol and 1024g of pure water, heat until completely dissolved, slowly cool at 20°C, continue to stir for 10h, and c...
Embodiment 3
[0075] A kind of preparation method of L-praziquantel of the present embodiment, other steps are identical with embodiment 2, just the basic compound of step (4) is triethylamine:
[0076] Add 194g of L-praziquantel amine tartrate to 1100g of dichloromethane; under stirring, add 100g of triethylamine dropwise, after the dropwise addition, stir for 30min, cool to below 7°C and dropwise add cyclohexanoyl chloride (51.4g) After a solution of dichloromethane (200g) was reacted at 3-5°C for 2h, the reaction was complete as detected by TLC. The stirring was turned off, and the layers were separated. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 110 g of crude L-praziquantel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com