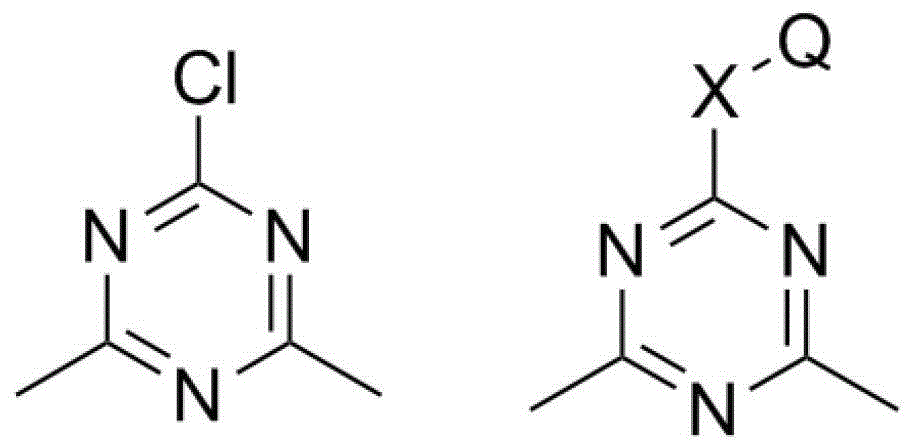
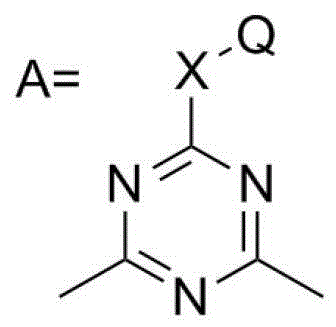
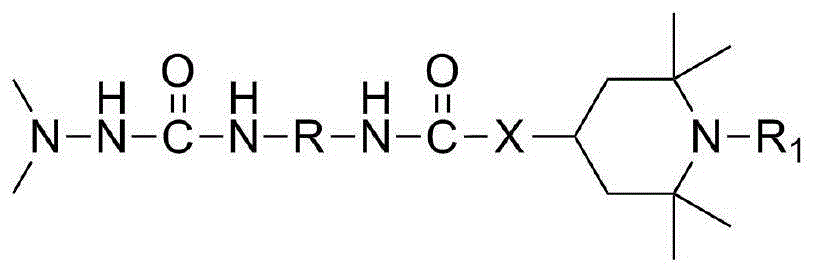Dimethylhydarzine stabilizer and stabilizer composition thereof
A technology of dimethyl hydrazine and stabilizer, applied in the field of stabilizer composition, can solve the problems such as the inability to improve the light resistance of organic polymer materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Embodiment 1: synthetic compound A
[0095] In a 500 ml four-neck mechanically stirred bottle, add 250 ml of toluene and 40 g (0.238 moles) of 1,6-hexyl diisocyanate under a nitrogen atmosphere, and slowly add 14.3 grams (0.2383 moles) of 1,1- Dimethylhydrazine, there will be exothermic phenomena during the dripping process, the temperature will naturally rise to 50-55°C, after one hour of reaction, add 37.3 grams of tetramethylpiperidinol, maintain the temperature at 55-60°C for three hours, and then heat up to 90°C, after two hours of reaction, the temperature was raised to 112°C to evaporate the toluene and the residual toluene was evaporated with a circulating water vacuum motor to obtain 89 g of a white solid. Compound structures and results are shown in Table (1).
Embodiment 2
[0096] Embodiment 2: synthetic compound B
[0097] In a 500 ml four-neck mechanical stirring bottle, add 250 ml of toluene and 50 g (0.2 moles) of 4,4'-methylene bis(phenylisocyanate) under nitrogen atmosphere, and slowly add 31.2 grams (0.2 moles) of tetramethylpiperidinamine, there will be exothermic phenomena during the dropping process, and the temperature will naturally rise to 40-45 ° C, after one hour of reaction, add 12.6 grams (0.21 moles) of 1,1-dimethyl Hydrazine, solids will be produced during the dropwise addition process, maintain the temperature at 55-60°C for three hours, then raise the temperature to 90°C, react for two hours and then cool down to 10°C to filter out the crystals to obtain 85 grams of light yellow solid. Compound structures and results are shown in Table (1).
Embodiment 3
[0098] Embodiment 3: synthetic compound C
[0099] In a 500 ml four-neck mechanical stirring bottle, add 250 ml of toluene and 47 g (0.188 moles) of 4,4'-methylene bis(phenylisocyanate) under nitrogen atmosphere, and slowly add 32.1 gram (0.188 moles) of pentamethylpiperidinol and 0.1 gram of stannous octoate (T9), there will be exothermic phenomena during the addition process, and the temperature will naturally rise to 40-45 ° C, and 11.5 grams (0.191 moles) will be added after two hours of reaction 1,1-Dimethylhydrazine, maintain the temperature at 55-60°C for three hours, then raise the temperature to 90°C, react for two hours and then raise the temperature to 112°C to evaporate the toluene and use a circulating water vacuum motor to evaporate the residual toluene to obtain 86 grams of white solid. Compound structures and results are shown in Table (1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com